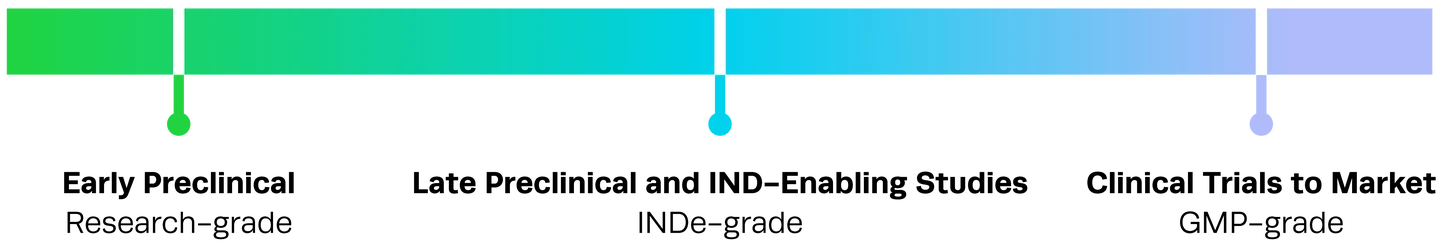
Synthego stands as a trusted partner, empowering researchers with innovative tools that meet the unique requirements of modern therapeutic development. Our comprehensive approach simplifies your complex workflows, delivering the highest-quality materials for exploratory studies and regulatory-grade solutions for clinical applications so you can seamlessly transition from discovery to clinic. Our proven track record in supporting 12 IND approvals speaks to the transformative potential of our products and the level of trust our clients place in us.
Explore the value of working with a proven, end-to-end CRISPR solutions provider, where innovation meets execution.