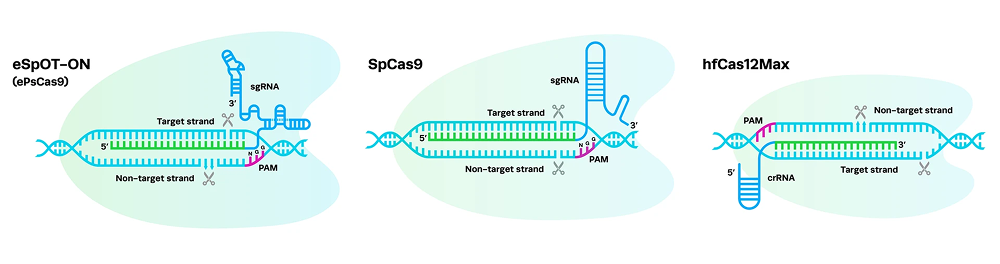
Learn more
Webcast featuring eSpOT-ON
Explore More
eSpOT-ON Nuclease Protein Available Now
Explore More
Order eSpOT-ON Nuclease mRNA Now