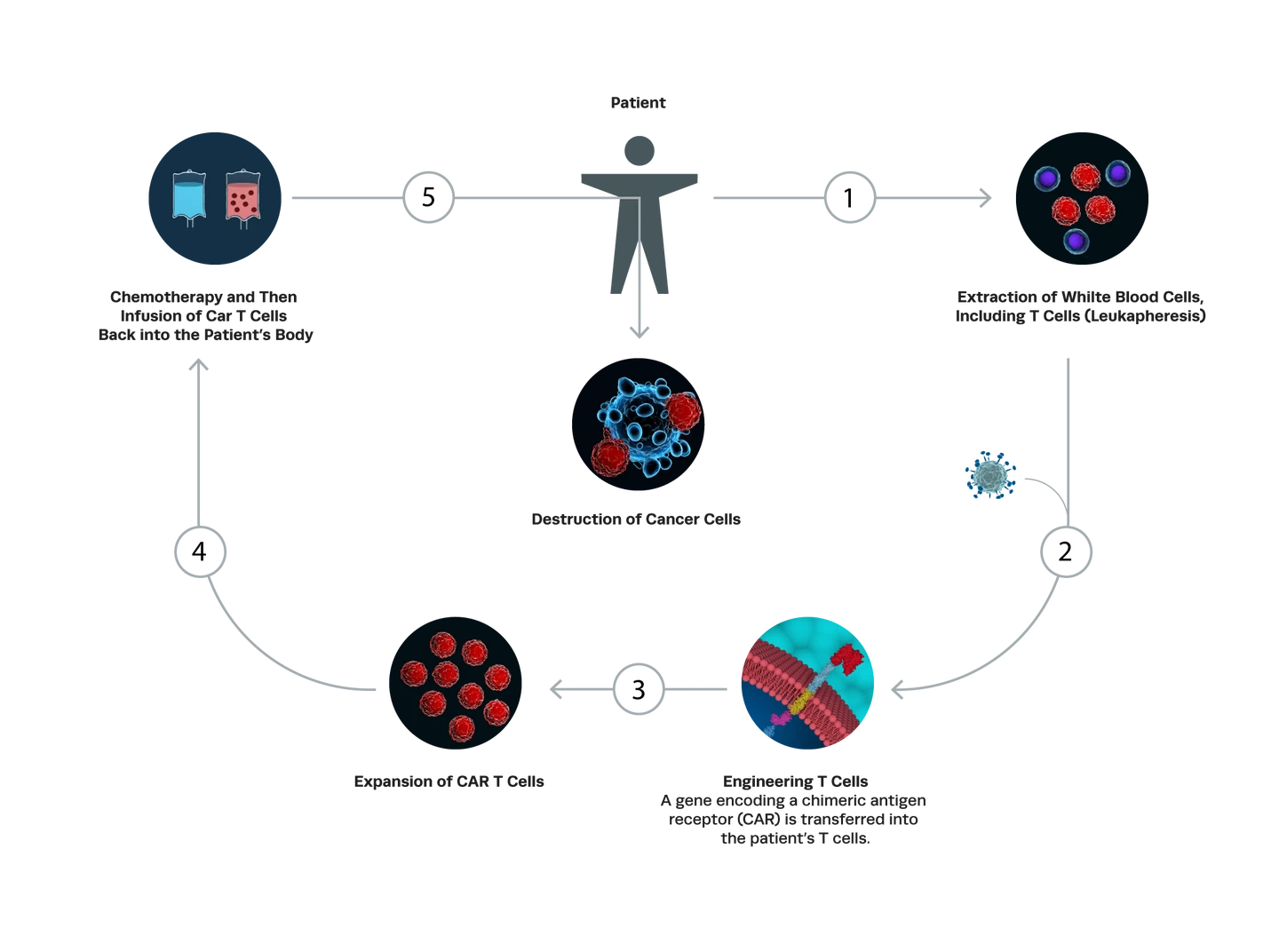
Chimeric antigen receptor T cells (CAR-T cells) are genetically modified T lymphocytes designed to recognize and eliminate tumor cells. These engineered T cells are derived from either the patient (autologous) or a healthy donor (allogeneic) and are modified to express chimeric antigen receptors, known as CARs, on the surface of the cell.
CARs are synthetic fusion proteins composed of:
- An extracellular single-chain variable fragment (scFv) that determines antigen specificity, usually derived from a monoclonal antibody
- A transmembrane domain
- Intracellular signaling domains responsible for T-cell activation, commonly including CD3ζ and costimulatory molecules such as CD28 or 4-1BB.
The unique structure of CARs enables T cells to identify and bind to tumor-associated antigens (TAAs) on the surface of cancer cells in an MHC-independent manner, enhancing their ability to target a broad range of tumor types.
This MHC-independence distinguishes CAR-T cells from conventional T-cell receptors (TCRs), which require antigen presentation via HLA molecules. The design of CARs allows for robust and direct cytotoxic activity against antigen-expressing tumor cells, as demonstrated in hematologic malignancies like B cell acute lymphoblastic leukemia (B-ALL) and diffuse large B cell lymphoma (DLBCL) (June et al., 2018, Science; Maude et al., 2014, NEJM).
While early CAR-T therapies relied on viral vectors for gene delivery, these methods often resulted in random transgene integration, potentially leading to variable CAR expression or unintended genomic disruptions. This means the CAR-T cell therapy could become less effective, or worse, less safe. To overcome these limitations, researchers turned to CRISPR to make precise, targeted modifications of T cells resulting in safer CAR-T therapies.