The long-held promise of cell and gene therapy is finally a reality. Recent improvements in our understanding of the human genome, combined with advances in genome engineering technology, have resulted in a groundswell of innovation in the cell and gene therapy field. As more of these therapies progress through clinical trials, we are beginning to see results that can transform patients' lives.
Cell and gene therapies have the power to not only treat the root cause of genetic disorders but also to dismantle tumors and tackle infectious diseases. The recent boom of gene therapies and gene-edited cell gene therapies is primarily owing to the advent of CRISPR technology, one of the key scientific breakthroughs of this century.
"Most gene therapy has focused on gene replacement until recently. CRISPR allows one to potentially approach gene therapy from the standpoint of editing: correcting mutations that cause disease."
Scott Burger, MD Principal for Advanced Cell and Gene Therapy
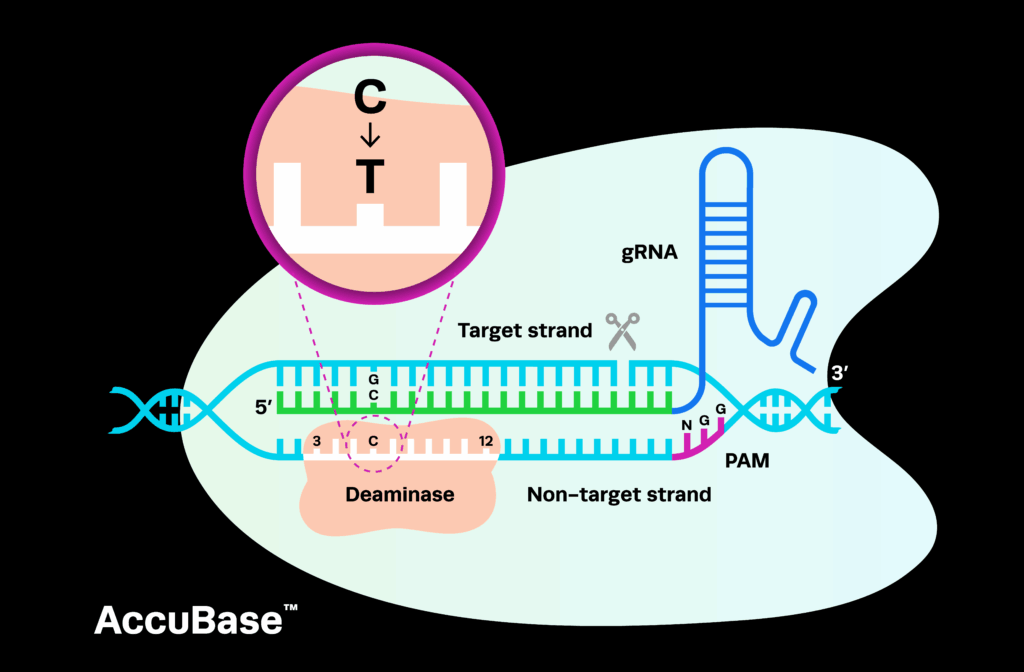
Acting as molecular scissors, CRISPR allows for the simple, precise, and customizable editing of DNA. By changing only a short sequence of guide RNA (gRNA), CRISPR can be used to edit almost anywhere in the genome, including the mutations that cause disease in humans. This amazing technology can create gene therapies, in which therapeutic editing takes place inside the body (in vivo), and gene-edited cell therapies, in which cells are edited outside the body (ex vivo) and then transplanted into patients.
CRISPR offers several advantages over previously available genome engineering technologies such as zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs). Not only is CRISPR more efficient at editing than other methods, but it is also simpler to perform, is more customizable, cost-effective, has a wider range of genomic targets, and can generate multiple genomic edits within a cell line or organism simultaneously, making it an ideal tool for generating cell therapies or treating complex genetic disorders.
CRISPR was first tested in human subjects in 2016 in a clinical trial of an ex vivo gene-edited cell therapy for non-small cell lung cancer (NSCLC). In 2019, a CRISPR gene therapy was tested in vivo for the first time in a clinical trial for the treatment of Leber’s congenital amaurosis (LCA). Today, CRISPR cell and gene therapy drugs are being tested in clinical trials for various genetic disorders, infectious diseases, and cancer.
Although CRISPR technology presents new questions for regulatory agencies, it has essentially democratized gene editing and enabled scientists to solve problems that have plagued the cell and gene therapy field since its inception. However, there remains a series of key obstacles that can prevent the successful translation of CRISPR cell and gene therapy products.
Key Challenges in CRISPR Cell and Gene Therapy Development
With the field set to expand at an unprecedented rate, eliminating obstacles in the development of CRISPR-based therapies is essential to get these therapies to patients as quickly and safely as possible. Synthego’s recent market research revealed the major challenges faced by cell and gene therapy researchers when progressing toward clinical trials, and, importantly, we also have solutions to many of these challenges that will help smooth your path to the clinic.
Regulatory hurdles for cell and gene therapy developmentThe FDA’s existing clinical development framework is a poor fit for the pace and scope of innovation in the CRISPR field. This is because the existing framework was developed for small molecule drug development, not for complex cell and gene therapies. CRISPR cell and gene therapy products present a new set of questions and concerns within a clinical framework: How can the sequence be confirmed? If a cell or gene therapy provides relief, how durable is that relief? What happens if mistakes are made?
Regulatory guidance continues to evolve, with the FDA constantly optimizing the frameworks and initiatives that will help streamline the production of cell and gene therapy drugs. For researchers in a clinical setting, knowing when to use GMP-grade materials, developing a thorough risk management plan, and understanding the required validation for CRISPR drug substances can be overwhelming and delay your path to the clinic without the most current and accurate information.
The FDA’s existing clinical development framework is a poor fit for the pace and scope of innovation in the CRISPR field. This is because the existing framework was developed for small molecule drug development, not for complex cell and gene therapies. CRISPR cell and gene therapy products present a new set of questions and concerns within a clinical framework: How can the sequence be confirmed? If a cell or gene therapy provides relief, how durable is that relief? What happens if mistakes are made?
Regulatory guidance continues to evolve, with the FDA constantly optimizing the frameworks and initiatives that will help streamline the production of cell and gene therapy drugs. For researchers in a clinical setting, knowing when to use GMP-grade materials, developing a thorough risk management plan, and understanding the required validation for CRISPR drug substances can be overwhelming and delay your path to the clinic without the most current and accurate information.
Synthego Solution
"At Synthego, we function as a partner throughout every stage of the clinical development process. By providing expert regulatory support, including aiding CMC modules for cell and gene therapy common technical documents (CTD), we can significantly improve your chances of a successful IND application. We can also support you in your interactions with the FDA, such as pre-IND meetings, accelerating your path to the clinic. "
Obtaining GMP reagents for cell and gene therapiesCRISPR cell and gene therapies primarily involve the Cas nuclease and the gRNA, in addition to any donor DNA that might be used for knocking in genes. Any cell and gene therapy product expected to enter clinical trials in human subjects must contain reagents that adhere to current Good Manufacturing Practice (cGMP) regulations, which ensure that the product is pure, safe, and effective.
Producing GMP-grade reagents requires scientific expertise, dedicated production facilities, controlled and authenticated cell lines and other materials, precision sequencing technology, stringent purity, and quality control testing, and extensive documentation. Obtaining high-quality GMP-grade gRNAs and nuclease is critical to ensure that CRISPR therapies are safe for patients and are able to move to clinical trials.
However, owing to the complexity of GMP requirements, there are relatively few companies that currently offer true GMP gRNAs, and the increase in cell and gene therapy products in development means the demand is rapidly outstripping supply. Many developers have encountered significant issues both with obtaining true GMP CRISPR reagents and not just "GMP-like," as well as the timely procurement of those GMP reagents.
CRISPR cell and gene therapies primarily involve the Cas nuclease and the gRNA, in addition to any donor DNA that might be used for knocking in genes. Any cell and gene therapy product expected to enter clinical trials in human subjects must contain reagents that adhere to current Good Manufacturing Practice (cGMP) regulations, which ensure that the product is pure, safe, and effective.
Producing GMP-grade reagents requires scientific expertise, dedicated production facilities, controlled and authenticated cell lines and other materials, precision sequencing technology, stringent purity, and quality control testing, and extensive documentation. Obtaining high-quality GMP-grade gRNAs and nuclease is critical to ensure that CRISPR therapies are safe for patients and are able to move to clinical trials.
However, owing to the complexity of GMP requirements, there are relatively few companies that currently offer true GMP gRNAs, and the increase in cell and gene therapy products in development means the demand is rapidly outstripping supply. Many developers have encountered significant issues both with obtaining true GMP CRISPR reagents and not just "GMP-like," as well as the timely procurement of those GMP reagents.
Synthego Solution
"Synthego’s state-of-the-art GMP facility ensures a secure supply chain of high-quality, GMP-grade sgRNAs, with ample capacity to maintain rigid clinical timelines for cell and gene therapy development. We are flexible so that you don’t have to compromise on your timeline, sequence, or quantity requirements. Get exactly what you need, when you need it. "
Ensuring consistency in cell and gene therapy developmentWhen a cell and gene therapy drug enters clinical trials, standardization is critical to ensure safety, efficacy, and regulatory approval. However, cell and gene therapies are inherently variable, and changing vendors of critical raw materials between research and clinical stages can result in unintended changes in the process because the reagents may not be equivalent. This can lead to clinical results that are not comparable, as well as presenting additional risks to patient safety.
Repeating preclinical research due to a discrepancy in materials could lead to months to years and millions of dollars lost, and may delay or prevent the therapy from being approved. Sticking with the same vendor from bench to clinic brings repeatable, reliable results, provided they have quality materials. Looking for a vendor that provides GMP-grade sgRNA and other materials reduces risks in the cell and gene therapy development pipeline.
When a cell and gene therapy drug enters clinical trials, standardization is critical to ensure safety, efficacy, and regulatory approval. However, cell and gene therapies are inherently variable, and changing vendors of critical raw materials between research and clinical stages can result in unintended changes in the process because the reagents may not be equivalent. This can lead to clinical results that are not comparable, as well as presenting additional risks to patient safety.
Repeating preclinical research due to a discrepancy in materials could lead to months to years and millions of dollars lost, and may delay or prevent the therapy from being approved. Sticking with the same vendor from bench to clinic brings repeatable, reliable results, provided they have quality materials. Looking for a vendor that provides GMP-grade sgRNA and other materials reduces risks in the cell and gene therapy development pipeline.
Synthego Solution
"Synthego provides a full continuum of CRISPR Solutions for each phase of CRISPR-based therapeutic development, from RUO-to-GMP. Consistently using a single vendor ensures cost-effectiveness while eliminating the need for quality variations, re-standardization requirements, and other hassles during your therapeutic development process. "
Finding experts for cell and gene therapy trialsClinical trials running over multiple years require highly experienced staff who work in concert to ensure the success of the trial. This includes a variety of specialist scientists, clinical and program project managers, clinical data managers and biostatisticians, clinical safety officers, quality and regulatory experts, a lawyer or legal team, and medical writers.
The complexity and novelty of CRISPR therapies in particular calls for extensive expertise, and the current boom in cell and gene therapy development has led to staff shortages that can impede the progress of clinical trials. When selecting a vendor for CRISPR reagents, researchers highly value expertise and experience so that they can be supported throughout the clinical development process.
Clinical trials running over multiple years require highly experienced staff who work in concert to ensure the success of the trial. This includes a variety of specialist scientists, clinical and program project managers, clinical data managers and biostatisticians, clinical safety officers, quality and regulatory experts, a lawyer or legal team, and medical writers.
The complexity and novelty of CRISPR therapies in particular calls for extensive expertise, and the current boom in cell and gene therapy development has led to staff shortages that can impede the progress of clinical trials. When selecting a vendor for CRISPR reagents, researchers highly value expertise and experience so that they can be supported throughout the clinical development process.
Synthego Solution
"As an industry leader, Synthego has unparalleled expertise in CRISPR technology. With 200+ batches of GMP gRNAs, 12 approved IND submissions supported, and 40+ successful customer audits, our CRISPR and regulatory experts understand the complexities of CRISPR-based therapies, and are here to partner with you so that your CRISPR therapy has the highest chance of success. "
Your CRISPR Guide to Clinical CRISPR Solutions
GMP sgRNA and GMP SpCas9 Nuclease for Advancing your CRISPR-Based Therapeutic
A New Vision for CRISPR Cell and Gene Therapies
Despite the significant challenges that researchers face during clinical development, we are already seeing CRISPR cell and gene therapy products transform the lives of patients. Check out our page on current CRISPR-based clinical trials.
With CRISPR’s ability to perform highly precise gene editing, the possibilities for cell and gene therapy are endless. We have entered an era of truly curative, personalized medicine. With the power of CRISPR, we can take control of our biology at a fundamental level to effect change, and there may soon be many more approved treatments that can reach large numbers of patients. Curious to learn more about how Synthego can help advance your clinical journey?