Cell and gene therapies have been around for some time, but it was only after the invention of CRISPR that we started seeing more clinical trials bringing in the hope of permanent cures for genetic diseases. In this blog post, we’ll explore the history of cell and gene therapies, the CRISPR revolution, the current state of play and recent boom in the CRISPR cell and gene therapy field, and the future of these life-changing treatments.
What is Cell and Gene Therapy?
Cell and gene therapies are a distinct group of medical technologies that aim to treat diseases through the transfer of either whole cells (cell therapy) or genetic material (gene therapy) into patients. While these technologies have some overlap, there are key differences between cell therapies and gene therapies.
Gene TherapyGene therapy refers to the introduction of new genetic material or the modification of a patient’s faulty genes associated with a particular disease. These modifications may be done directly inside the patient’s body (in vivo) or by editing cells outside of the body (ex vivo). There are over 5,000 known monogenic diseases, meaning that they are caused by mutations in a single gene. In these cases, correcting the mutated gene or delivering a functional copy of the gene can alleviate the condition.
Gene therapy refers to the introduction of new genetic material or the modification of a patient’s faulty genes associated with a particular disease. These modifications may be done directly inside the patient’s body (in vivo) or by editing cells outside of the body (ex vivo). There are over 5,000 known monogenic diseases, meaning that they are caused by mutations in a single gene. In these cases, correcting the mutated gene or delivering a functional copy of the gene can alleviate the condition.
Cell TherapyCell therapy involves transfusing a patient with healthy cells to compensate for a lack of certain cells or to replace diseased cells. Cell therapy can be either autologous, meaning that the cells used are derived from the patient, or allogeneic, meaning that they are derived from healthy donors. Gene-edited cell therapy involves removing cells from patients and editing them ex vivo to either correct disease-causing mutations, or add new genes that will make them more robust for treating disease. Common cell types used in this type of therapy are hematopoietic stem and progenitor cells (HSPCs), induced pluripotent stem cells, and immune cells.
Cell therapy involves transfusing a patient with healthy cells to compensate for a lack of certain cells or to replace diseased cells. Cell therapy can be either autologous, meaning that the cells used are derived from the patient, or allogeneic, meaning that they are derived from healthy donors. Gene-edited cell therapy involves removing cells from patients and editing them ex vivo to either correct disease-causing mutations, or add new genes that will make them more robust for treating disease. Common cell types used in this type of therapy are hematopoietic stem and progenitor cells (HSPCs), induced pluripotent stem cells, and immune cells.
First Steps: Cell Therapy
Cell therapies preceded gene therapies by several decades. Let’s take a cool at the first attempts at cell therapy and how different types of cell therapies emerged over time.
Blood transfusions and bone marrow transplantationTechnically speaking, blood transfusions were the first cell therapies. After early attempts failed, the discovery of blood types allowed for the successful transfer of whole blood from compatible donors via IV infusion. It wasn’t until the late 1950s that the first hematopoietic stem cell transplant (HSCT) was attempted. Despite initial failures, HSCT is now the standard of care for blood disorders, including cancer.
Technically speaking, blood transfusions were the first cell therapies. After early attempts failed, the discovery of blood types allowed for the successful transfer of whole blood from compatible donors via IV infusion. It wasn’t until the late 1950s that the first hematopoietic stem cell transplant (HSCT) was attempted. Despite initial failures, HSCT is now the standard of care for blood disorders, including cancer.
Embryonic stem cellsThe 1990s saw a key breakthrough in the nascent field of regenerative medicine: researchers had successfully isolated stem cells from a human embryo and managed to grow them in the lab while retaining their pluripotent state. But despite their initial promise, embryonic stem cells fell short of the mark; they were plagued by immune rejection and differentiation issues, and were the subject of intense ethical debates which resulted in their experimental use being prohibited in many countries.
The 1990s saw a key breakthrough in the nascent field of regenerative medicine: researchers had successfully isolated stem cells from a human embryo and managed to grow them in the lab while retaining their pluripotent state. But despite their initial promise, embryonic stem cells fell short of the mark; they were plagued by immune rejection and differentiation issues, and were the subject of intense ethical debates which resulted in their experimental use being prohibited in many countries.
Induced pluripotent stem cellsFinally, in 2006, the development of induced pluripotent stem cells (iPSCs) led to renewed interest in regenerative medicine. By discovering key pluripotency-related transcription factors, Dr. Shinya Yamanaka was able to reprogram adult cells - like skin or blood cells - back into a pluripotent state. These iPSCs closely resembled embryonic stem cells and could be used to create almost any type of cell, while avoiding ethical and immune rejection issues associated with their embryonic counterparts.
Finally, in 2006, the development of induced pluripotent stem cells (iPSCs) led to renewed interest in regenerative medicine. By discovering key pluripotency-related transcription factors, Dr. Shinya Yamanaka was able to reprogram adult cells - like skin or blood cells - back into a pluripotent state. These iPSCs closely resembled embryonic stem cells and could be used to create almost any type of cell, while avoiding ethical and immune rejection issues associated with their embryonic counterparts.
The Early Days of Gene Therapy
When the idea of gene therapy was first proposed, it seemed like science fiction - even to medical professionals and researchers. Let’s explore the emergence of gene therapy, its promise, and early setbacks.
Delivering functional genesThe 1960s saw several key scientific developments that laid the foundations for gene therapy and gene-edited cell therapies, including the first incorporation of DNA into human cells, the use of viruses to deliver DNA, and the correction of genetic mutations in cultured cells. However, the concept of gene therapy was first suggested in the 1970s, a decade that saw the introduction of both recombinant DNA technology and DNA sequencing. The earliest gene therapies relied on delivering a functional copy of faulty genes using viral vectors.
The 1960s saw several key scientific developments that laid the foundations for gene therapy and gene-edited cell therapies, including the first incorporation of DNA into human cells, the use of viruses to deliver DNA, and the correction of genetic mutations in cultured cells. However, the concept of gene therapy was first suggested in the 1970s, a decade that saw the introduction of both recombinant DNA technology and DNA sequencing. The earliest gene therapies relied on delivering a functional copy of faulty genes using viral vectors.
The first successful gene therapyThe first successful gene therapy was administered on September 15, 1990, when a four-year-old girl suffering from a rare immune disorder, adenosine deaminase deficiency, received functional copies of the recombinant ADA gene. This form of gene therapy is not a permanent fix, and the patient in question required multiple subsequent treatments. However, this key breakthrough in the gene therapy field generated excitement and optimism for patients and the scientific community alike.
The first successful gene therapy was administered on September 15, 1990, when a four-year-old girl suffering from a rare immune disorder, adenosine deaminase deficiency, received functional copies of the recombinant ADA gene. This form of gene therapy is not a permanent fix, and the patient in question required multiple subsequent treatments. However, this key breakthrough in the gene therapy field generated excitement and optimism for patients and the scientific community alike.
"This is a dramatic event in medical history... We've been waiting for years for the day when we could introduce a working gene to replace a gene that doesn't function properly, and I'm delighted it's finally happening."
Dr. Charles J. Epstein, UCSF pediatrician, member of the panel that approved the first experimental gene therapy to treat ADA deficiency, September 1990. Source: The New York Times.
A major setbackAfter some initial success, a major setback to the gene therapy field came in 1999. Jessie Gelsinger, a young man suffering from ornithine transcarbamylase deficiency syndrome (OTCD), a rare metabolic disorder, died from complications arising from his experimental treatment. This was likely the result of poor liver function, which is now commonly used as an exclusion criterion for gene therapy trials, or a severe immune reaction to the high dose of adenovirus used to deliver the gene.
After some initial success, a major setback to the gene therapy field came in 1999. Jessie Gelsinger, a young man suffering from ornithine transcarbamylase deficiency syndrome (OTCD), a rare metabolic disorder, died from complications arising from his experimental treatment. This was likely the result of poor liver function, which is now commonly used as an exclusion criterion for gene therapy trials, or a severe immune reaction to the high dose of adenovirus used to deliver the gene.
Success in Gene Replacement Therapy and Gene Editing
Researchers didn’t entirely give up on the idea of gene therapy after those early setbacks. They kept chipping away at it, and they made some key breakthroughs in the next two decades.
Gene therapy makes a comeback Over a decade after Jessie Gelsinger’s death, with the development of better vectors for gene delivery, the field began to see renewed interest and excitement. In 2003, modified lentiviruses were used to deliver gene replacement therapies for the first time, and the first commercially available gene therapy, Gendicine, was released in China to treat cancer.
Over a decade after Jessie Gelsinger’s death, with the development of better vectors for gene delivery, the field began to see renewed interest and excitement. In 2003, modified lentiviruses were used to deliver gene replacement therapies for the first time, and the first commercially available gene therapy, Gendicine, was released in China to treat cancer.
Gene-edited cell therapy emergesThe discovery of zinc finger nucleases (ZFNs) in 2005, followed by transcription activator-like effector nucleases (TALENs) in 2011, made the dream of actually rewriting human genes a reality. By editing cells ex vivo, researchers could create better, stronger cell therapies for a variety of diseases, including cancer. In 2014, ZFNs were used to treat HIV/AIDS by engineering patient-derived T cells, knocking out the CCR5 gene which is a coreceptor of the virus. Soon after, TALENs offered another addition to the genome-editing toolkit and were used to engineer T cells to fight B-cell cancers.
Despite several key breakthroughs in this time period, progress was still relatively slow - patients required ongoing treatments to achieve results with virus-delivered gene replacement therapies, and the ZFN and TALEN gene-editing systems were fairly blunt tools that required significant time and cost investments. It seemed like an uphill battle, but scientists had no idea the world of gene and cell therapy was about to change forever.
The discovery of zinc finger nucleases (ZFNs) in 2005, followed by transcription activator-like effector nucleases (TALENs) in 2011, made the dream of actually rewriting human genes a reality. By editing cells ex vivo, researchers could create better, stronger cell therapies for a variety of diseases, including cancer. In 2014, ZFNs were used to treat HIV/AIDS by engineering patient-derived T cells, knocking out the CCR5 gene which is a coreceptor of the virus. Soon after, TALENs offered another addition to the genome-editing toolkit and were used to engineer T cells to fight B-cell cancers.
Despite several key breakthroughs in this time period, progress was still relatively slow - patients required ongoing treatments to achieve results with virus-delivered gene replacement therapies, and the ZFN and TALEN gene-editing systems were fairly blunt tools that required significant time and cost investments. It seemed like an uphill battle, but scientists had no idea the world of gene and cell therapy was about to change forever.
The CRISPR Revolution
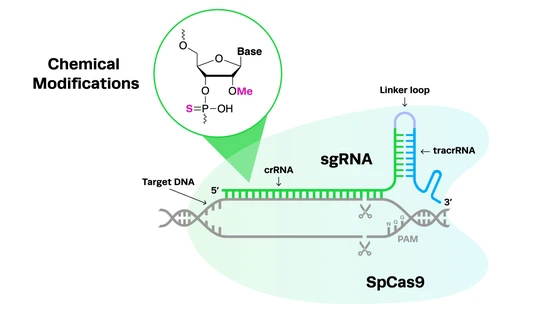
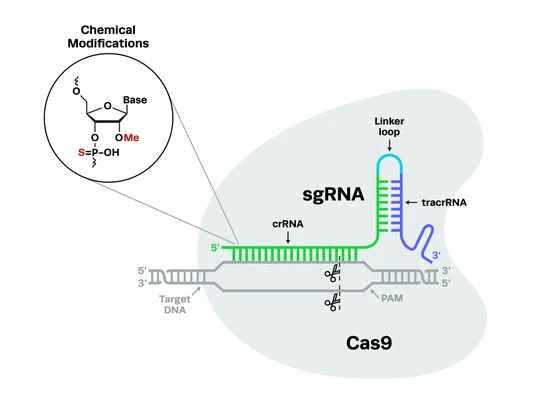
The development of CRISPR-Cas9 as an easily customizable gene-editing tool in 2012 was groundbreaking for gene therapy researchers. CRISPR can fix the root cause of genetic conditions, potentially curing disease with a single treatment. Compared to previous gene-editing methods, CRISPR-Cas9 is also much simpler to perform, more precise and efficient at editing, and more cost-effective.
The simplicity of the CRISPR-Cas system and its easily programmable guides means that researchers can easily adapt it to any of the thousands of potential disease targets. It has predictable and consistent outcomes, with precise editing and low off-target activity compared to previous technologies, offering rapid translation from the bench to the clinic and higher levels of safety for patients.
The first CRISPR cell and gene therapies enter the clinicThe progress in cell and gene therapies since the discovery of CRISPR has been rapid. The first trial of an ex vivo CRISPR T cell therapy for non-small-cell lung cancer (NSCLC) began in 2016, a mere four years after CRISPR was invented. In 2018, a CRISPR cell therapy trial for sickle cell disease (SCD) began, editing the cells of SCD patients to restore production of fetal hemoglobin. The first in vivo CRISPR gene therapy trials began in 2019, treating a patient with Leber’s congenital amaurosis (LCA) by correcting a mutation in the CEP290 gene.
CRISPR has enabled immune cells, such as T cells, to be genetically modified so that they become much more efficient at fighting cancer and other diseases. Examples of gene-edited cell therapies include chimeric antigen receptor (CAR) T cell therapy, modified T cell receptor (TCR) cell therapy, natural killer (NK) cell therapy, and tumor-infiltrating lymphocyte (TIL) cell therapy.
Scientists are also working to develop CRISPR gene therapies and gene-edited cell therapies for a wide range of monogenic diseases, including blood disorders, neurodegenerative disorders, hereditary blindness, and immune disorders. For more information on these possibilities, you can read our blog exploring diseases CRISPR can potentially cure and the latest in CRISPR clinical trials. CRISPR gene therapies are not limited to DNA editing for the treatment of genetic diseases and cancer - they are also currently being developed for infectious diseases, such as HIV.
The progress in cell and gene therapies since the discovery of CRISPR has been rapid. The first trial of an ex vivo CRISPR T cell therapy for non-small-cell lung cancer (NSCLC) began in 2016, a mere four years after CRISPR was invented. In 2018, a CRISPR cell therapy trial for sickle cell disease (SCD) began, editing the cells of SCD patients to restore production of fetal hemoglobin. The first in vivo CRISPR gene therapy trials began in 2019, treating a patient with Leber’s congenital amaurosis (LCA) by correcting a mutation in the CEP290 gene.
CRISPR has enabled immune cells, such as T cells, to be genetically modified so that they become much more efficient at fighting cancer and other diseases. Examples of gene-edited cell therapies include chimeric antigen receptor (CAR) T cell therapy, modified T cell receptor (TCR) cell therapy, natural killer (NK) cell therapy, and tumor-infiltrating lymphocyte (TIL) cell therapy.
Scientists are also working to develop CRISPR gene therapies and gene-edited cell therapies for a wide range of monogenic diseases, including blood disorders, neurodegenerative disorders, hereditary blindness, and immune disorders. For more information on these possibilities, you can read our blog exploring diseases CRISPR can potentially cure and the latest in CRISPR clinical trials. CRISPR gene therapies are not limited to DNA editing for the treatment of genetic diseases and cancer - they are also currently being developed for infectious diseases, such as HIV.
CRISPR broadens its scope: New nucleases, epigenetic editing, and moreOther recent CRISPR breakthroughs include the discovery of new Cas nucleases which are more precise or better suited for specific purposes, such as RNA editing enzymes like Cas7-11, allowing for CRISPR cell and gene therapies to be developed for a wider variety of diseases. Similar developments in CRISPR-based epigenetic editing have opened new doors for the treatment of diseases that are affected by epigenetic conditions, including cancer, and could help control the activity of cell therapies.
New CRISPR applications, like base editing and prime editing techniques, which do not induce double-stranded breaks in DNA, offer safer and more precise treatments. There has also been considerable recent progress made in validating the safety, quality, and efficacy of CRISPR cell and gene therapies, including better delivery vehicles for CRISPR-Cas9 gene-editing components, such as nanoparticles and modified viral vectors.
Other recent CRISPR breakthroughs include the discovery of new Cas nucleases which are more precise or better suited for specific purposes, such as RNA editing enzymes like Cas7-11, allowing for CRISPR cell and gene therapies to be developed for a wider variety of diseases. Similar developments in CRISPR-based epigenetic editing have opened new doors for the treatment of diseases that are affected by epigenetic conditions, including cancer, and could help control the activity of cell therapies.
New CRISPR applications, like base editing and prime editing techniques, which do not induce double-stranded breaks in DNA, offer safer and more precise treatments. There has also been considerable recent progress made in validating the safety, quality, and efficacy of CRISPR cell and gene therapies, including better delivery vehicles for CRISPR-Cas9 gene-editing components, such as nanoparticles and modified viral vectors.
Approval of the first CRISPR therapyJust a decade after the discovery of CRISPR gene editing technology, it has already changed the lives of patients in the clinic. In 2023, researchers celebrated the approval of the first CRISPR therapy for widespread use; Casgevy, also known as exa-cel, was approved by the FDA for the treatment of SCD after it successfully completed clinical trials.
The autologous gene-edited cell therapy uses CRISPR-Cas9 technology to edit the BCL11A gene in patient CD34+ hematopoietic stem cells, resulting in increased fetal hemoglobin production. Casgevy offers patients with SCD a functional cure, reducing or eliminating their vaso-occlusive crises. This landmark moment for CRISPR cell and gene therapies set a strong precedent for the development and approval of more genomic medicines.
Just a decade after the discovery of CRISPR gene editing technology, it has already changed the lives of patients in the clinic. In 2023, researchers celebrated the approval of the first CRISPR therapy for widespread use; Casgevy, also known as exa-cel, was approved by the FDA for the treatment of SCD after it successfully completed clinical trials.
The autologous gene-edited cell therapy uses CRISPR-Cas9 technology to edit the BCL11A gene in patient CD34+ hematopoietic stem cells, resulting in increased fetal hemoglobin production. Casgevy offers patients with SCD a functional cure, reducing or eliminating their vaso-occlusive crises. This landmark moment for CRISPR cell and gene therapies set a strong precedent for the development and approval of more genomic medicines.
Casgevy Approval Retrospective with Lina Jamis
Learn more about the groundbreaking approval of the US' first CRISPR therapy by Vertex Pharmaceuticals and CRISPR Therapeutics, and how it's reshaping the landscape of CRISPR regulations and ongoing clinical trials. In this insightful video, Lina Jamis, Associate Director of Regulatory Affairs at Synthego, shares key learnings and valuable perspectives on the latest developments in the field of CRISPR therapeutics. Stay informed and gain valuable insights into the future of CRISPR therapies.

Promising Future of CRISPR Cell and Gene Therapies
While it’s clear that progress in cell and gene therapy since the invention of CRISPR has been rapid, we’ve only begun to scratch the surface of the true potential of CRISPR technology. Since the approval of Casgevy, many more CRISPR gene editing therapies and gene-edited cell therapies have entered the clinic.
These include therapies for the treatment of genetic disorders like diabetes, muscular dystrophies, cardiovascular disease, and chronic granulomatosis, as well as infectious diseases and a range of solid tumors and hematological cancers. With corresponding updates to the regulatory frameworks that govern these medicines, we hope to see many of these CRISPR medicines approved in the coming years.
Early 2025 saw another critical breakthrough for CRISPR therapies: the creation of an on-demand, personalized CRISPR medicine for an infant with a fatal mutation. Collaborating with researchers from UC Berkeley’s Innovative Genomics Institute (IGI), a team of physicians created a bespoke gene therapy to correct a mutation that causes carbamoyl phosphate synthetase 1 (CPS1) deficiency, which affects one in 1.3 million newborns.
From start to finish, the curative treatment took six months to produce, and the recipient, KJ, is now thriving. This example shows the power of CRISPR to rapidly produce personalized medicines for N=1 diseases, a concept championed by Fyodor Urnov, a CRISPR pioneer and the IGI’s Director of Technology and Translation. We hope to see many more.
The life-changing effects of cell and gene therapies are largely thanks to the development of CRISPR technology. CRISPR cell and gene therapies have the unique potential to address the root cause of genetic disease, and their platform potential allows the development of standardized processes, enabling the targeting of thousands of disease-causing mutations while ensuring the correct balance of safety, quality, and efficacy.
CRISPR technology is also constantly evolving; over the past decade, we have seen better quality programmable guides with improved predictability and higher control, which are key for ensuring safety in CRISPR therapies. At Synthego, we have licensed two new nucleases that have powerful potential in the clinic: eSPOT-On and hfCas12max. These nucleases are designed for use in the development of CRISPR cell and gene therapies, with robust editing efficiencies and strong safety profiles that will ensure clinical success.
Unlocking the true potential of CRISPR in the cell and gene therapy revolution in the future will require industrialization that enables rapid translation and simplified scalability with consistent manufacturing. In this regard, Synthego is poised to deliver GMP-grade sgRNAs at unprecedented scale and quality, supporting researchers from bench to clinic. With a stronger focus on safety and consistency, and the development of new tools, the growth of the CRISPR cell and gene therapy field will continue well into the future.
We hope this article helped you grasp some of the fascinating history of cell and gene therapies and the current state of play in the CRISPR cell and gene therapy field. The considerable recent acceleration in CRISPR gene therapies and gene-edited cell therapies, and an uptick in CRISPR biotech startups developing these treatments, means that an ever-increasing number of diseases can hopefully be cured over the coming years.