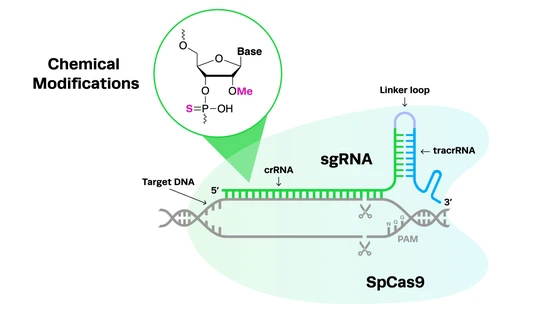
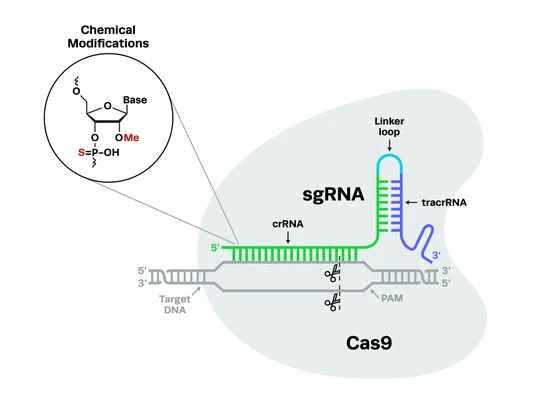
CRISPR technology transformed what you can achieve in genome editing. The original system, driven by the SpCas9 nuclease from Streptococcus pyogenes, introduced groundbreaking precision that opened doors to curing genetic diseases, fighting cancer, and tackling infectious diseases. But why limit yourself to SpCas9? The evolution of CRISPR has led to a variety of alternative and engineered nucleases designed to tackle unique challenges and expand the bounds of CRISPR-based therapies.
Why Use Alternatives to CRISPR-Cas9?
While SpCas9 is the most popular and well-characterized CRISPR nuclease, it’s not without its drawbacks. One significant concern lies in its tendency to create double-stranded breaks (DSBs) in DNA, which can lead to unintended deletions or rearrangements, potentially causing harmful mutations in the genome. Another challenge is the risk of off-target editing, raising safety concerns for therapeutic use. Additionally, the large size of SpCas9 presents a logistical hurdle, as it exceeds the packaging capacity of many delivery vehicles, like adeno-associated viruses (AAVs). These limitations have prompted researchers to explore novel nucleases, which minimize the risk of these issues and result in safer CRISPR-based therapies.
Synthego's CRISPR Nucleases
With a successful track record in synthesizing and optimizing gRNAs, Synthego is dedicated to equipping you with high-performance nucleases precisely paired with engineered gRNAs to meet your most challenging CRISPR objectives.

Synthego’s Cutting-Edge Engineered Nucleases
When you are developing new therapies, you deserve tools that work as hard as you do. Synthego’s engineered nucleases meet your therapeutic development needs with unmatched precision and performance. Paired with gRNAs that are optimized for RNP stability, these nucleases enable you to create safer, more effective in vivo and ex vivo cell and gene therapies.
hfCas12MaxhfCas12Max sets a new standard in clinical gene editing, offering a compact design paired with staggered-end cut outcomes that enhance precision and versatility. Its multiplexing capabilities and robust, highly specific editing performance ensure reliable results, while its broad PAM recognition profile significantly expands target accessibility. Compared to SaCas9 and Cas12a, hfCas12Max delivers superior adaptability and efficiency, making it the optimal choice for cutting-edge clinical applications.
hfCas12Max sets a new standard in clinical gene editing, offering a compact design paired with staggered-end cut outcomes that enhance precision and versatility. Its multiplexing capabilities and robust, highly specific editing performance ensure reliable results, while its broad PAM recognition profile significantly expands target accessibility. Compared to SaCas9 and Cas12a, hfCas12Max delivers superior adaptability and efficiency, making it the optimal choice for cutting-edge clinical applications.
eSpOT-ONeSpOT-ON (ePsCas9) delivers high on-target precision, extremely low off-target editing, recognizes the NGG PAM sequence, and staggered-end cuts that minimize translocation risks, ensuring safer and more reliable gene editing. As a recombinant protein or mRNA, eSpOT-ON is a superior option for developing effective single-dose therapies compared to traditional and high-fidelity Cas9 nucleases currently on the market.
eSpOT-ON (ePsCas9) delivers high on-target precision, extremely low off-target editing, recognizes the NGG PAM sequence, and staggered-end cuts that minimize translocation risks, ensuring safer and more reliable gene editing. As a recombinant protein or mRNA, eSpOT-ON is a superior option for developing effective single-dose therapies compared to traditional and high-fidelity Cas9 nucleases currently on the market.
Overcoming Off-Target Editing with Precision Nucleases
When you’re developing a CRISPR-based therapy, precision is critical. Off-target editing can jeopardize all your hard work, introducing mutations in unintended parts of the genome. These errors aren’t just frustrating; they can compromise the safety and efficacy of your therapy. That’s why achieving both high on-target editing accuracy and low off-target effects needs to be a top priority in your therapeutic designs.
The Solutions
- hfCas12Max: With its high specificity and broad TN or TTN PAM recognition, hfCas12Max offers you exceptional on-target editing efficiency with lower off-target cleavage. In fact, hfCas12Max has demonstrated more robust on-target editing and lower off-target editing than SpCas9 or other Cas12 variants in primary human T-cells and mice. This makes it ideal for applications where precision is critical, such as CAR-T, T-cell therapy, or gene therapies.
- eSpOT-ON (ePsCas9): Originally identified in the genome of the bacterium Parasutterella secunda, the wild-type SpotOn (PsCas9) already displayed high editing efficiency and was engineered to increase its on-target versus off-target activity. Unlike engineered high-fidelity SpCas9 variants, in which lower off-target editing comes at the cost of reduced on-target activity, eSpOT-ON provides you with the perfect balance of high on-target activity while maintaining reduced off-target effects. In fact, eSpOT-ON demonstrates high levels of editing with only a single administration when delivered to the liver of mice in LNPs.
- SaCas9: While the original SpCas9 recognizes an NGG PAM sequence, SaCas9 (from Staphylococcus aureus) recognizes an NNGRRN PAM, where R can be either an A or a G residue. Engineered SaCas9 variants have also been produced with broader PAM recognition. Optimized variants of SaCas9, with broader PAM recognition, help you limit off-target activity by offering flexibility in target site selection while maintaining robust editing capabilities.
- hfCas12Max: With its high specificity and broad TN or TTN PAM recognition, hfCas12Max offers you exceptional on-target editing efficiency with lower off-target cleavage. In fact, hfCas12Max has demonstrated more robust on-target editing and lower off-target editing than SpCas9 or other Cas12 variants in primary human T-cells and mice. This makes it ideal for applications where precision is critical, such as CAR-T, T-cell therapy, or gene therapies.
- eSpOT-ON (ePsCas9): Originally identified in the genome of the bacterium Parasutterella secunda, the wild-type SpotOn (PsCas9) already displayed high editing efficiency and was engineered to increase its on-target versus off-target activity. Unlike engineered high-fidelity SpCas9 variants, in which lower off-target editing comes at the cost of reduced on-target activity, eSpOT-ON provides you with the perfect balance of high on-target activity while maintaining reduced off-target effects. In fact, eSpOT-ON demonstrates high levels of editing with only a single administration when delivered to the liver of mice in LNPs.
- SaCas9: While the original SpCas9 recognizes an NGG PAM sequence, SaCas9 (from Staphylococcus aureus) recognizes an NNGRRN PAM, where R can be either an A or a G residue. Engineered SaCas9 variants have also been produced with broader PAM recognition. Optimized variants of SaCas9, with broader PAM recognition, help you limit off-target activity by offering flexibility in target site selection while maintaining robust editing capabilities.
Addressing Double-Stranded Break Challenges
Double-stranded breaks (DSBs) are at the heart of CRISPR editing, but for you, every cut carries risks. Unintended DSBs can result in deletions, chromosomal translocations, and mutations that threaten the integrity of your project. You need an alternative that reduces these risks and gives you tighter control over the process, so you can build safer, more reliable CRISPR therapies.
The Solutions
- eSpOT-ON: Unlike SpCas9, eSpOT-ON forms staggered-end DSBs rather than blunt-end cuts. Its cutting pattern reduces the likelihood of chromosomal translocations by creating DNA breaks that are more accurately repaired by the cell’s natural repair mechanisms. This precise repair process is crucial for maintaining genomic stability, improving its safety profile.
- hfCas12Max: Similar to eSpOT-ON, hfCas12Max creates staggered-end DSBs, which decrease the risk of unintended DSBs. These overhangs provide a specific guide for proper alignment of the DNA strands during repair, lowering the likelihood of incorrect chromosome connections. This accurate repair mechanism preserves genomic integrity and ensures safer gene-editing practices.
- dCas9: Catalytically dead Cas9 doesn’t make DSBs at all - its catalytic domain has been disabled. However, it retains its ability to search the genome for a target and bind to it. This means it can be fused to other useful proteins and enzymes for various purposes, directing them to the correct target. It has been adapted for applications such as CRISPR inhibition (CRISPRi) and CRISPR activation (CRISPRa), base editing, and even epigenome editing, where precision is required without cutting DNA.
- nCas9: The nickase version of Cas9 introduces single-stranded breaks instead of DSBs, making it a valuable tool for prime editing
- eSpOT-ON: Unlike SpCas9, eSpOT-ON forms staggered-end DSBs rather than blunt-end cuts. Its cutting pattern reduces the likelihood of chromosomal translocations by creating DNA breaks that are more accurately repaired by the cell’s natural repair mechanisms. This precise repair process is crucial for maintaining genomic stability, improving its safety profile.
- hfCas12Max: Similar to eSpOT-ON, hfCas12Max creates staggered-end DSBs, which decrease the risk of unintended DSBs. These overhangs provide a specific guide for proper alignment of the DNA strands during repair, lowering the likelihood of incorrect chromosome connections. This accurate repair mechanism preserves genomic integrity and ensures safer gene-editing practices.
- dCas9: Catalytically dead Cas9 doesn’t make DSBs at all - its catalytic domain has been disabled. However, it retains its ability to search the genome for a target and bind to it. This means it can be fused to other useful proteins and enzymes for various purposes, directing them to the correct target. It has been adapted for applications such as CRISPR inhibition (CRISPRi) and CRISPR activation (CRISPRa), base editing, and even epigenome editing, where precision is required without cutting DNA.
- nCas9: The nickase version of Cas9 introduces single-stranded breaks instead of DSBs, making it a valuable tool for prime editing
Tackling Gene Knock-In and Knockout Efficiency
Achieving efficient gene knock-ins and knockouts is likely a central part of your therapeutic goals. But it’s not always straightforward. Whether it’s correcting a faulty gene or inactivating a harmful one, the challenge lies in working within the genome’s natural repair mechanisms. The tools you choose can directly affect your ability to deliver effective treatments.
The Solutions
- hfCas12Max: Unlike SpCas9, hfCas12Max creates staggered-end or ‘sticky’ cuts, increasing the efficiency of HDR. With broad PAM recognition, hfCas12Max can target more areas in the genome, including AT-rich regions. This nuclease allows for multiplexed gene editing and has also been adapted as a tool for base editing applications, making hfCas12Max a powerful tool for gene knock-in applications.
- eSpOT-ON: While it recognizes the same NGG PAM sequence of SpCas9, eSpOT-ON forms staggered-end DSBs rather than blunt-end cuts. By creating three-nucleotide 5’ overhangs, eSpOT-ON is ideal for HDR-based applications, promoting safer and more reliable editing outcomes.
- Cas12a (Cpf1): Similar to hfCas12Max, Cas12a enhances HDR efficiency by creating staggered-end cuts, promoting successful gene knock-in. Cas12a can also be used to target AT-rich regions of the genome - something SpCas9 cannot.
- Cas3: Unique among nucleases, once Cas3 recognizes its target, it shreds large sections of DNA, up to 10kb in size. This makes it ideal for gene knockout because it is more likely to yield a complete functional knockout. Cas3 also lacks any PAM recognition requirements, so it has a much broader range of genomic targets.
- hfCas12Max: Unlike SpCas9, hfCas12Max creates staggered-end or ‘sticky’ cuts, increasing the efficiency of HDR. With broad PAM recognition, hfCas12Max can target more areas in the genome, including AT-rich regions. This nuclease allows for multiplexed gene editing and has also been adapted as a tool for base editing applications, making hfCas12Max a powerful tool for gene knock-in applications.
- eSpOT-ON: While it recognizes the same NGG PAM sequence of SpCas9, eSpOT-ON forms staggered-end DSBs rather than blunt-end cuts. By creating three-nucleotide 5’ overhangs, eSpOT-ON is ideal for HDR-based applications, promoting safer and more reliable editing outcomes.
- Cas12a (Cpf1): Similar to hfCas12Max, Cas12a enhances HDR efficiency by creating staggered-end cuts, promoting successful gene knock-in. Cas12a can also be used to target AT-rich regions of the genome - something SpCas9 cannot.
- Cas3: Unique among nucleases, once Cas3 recognizes its target, it shreds large sections of DNA, up to 10kb in size. This makes it ideal for gene knockout because it is more likely to yield a complete functional knockout. Cas3 also lacks any PAM recognition requirements, so it has a much broader range of genomic targets.
Meeting Delivery Challenges in Therapeutic Applications
Designing your CRISPR framework to align seamlessly with your delivery approach is key to achieving therapeutic success, but navigating delivery constraints can be challenging. Delivery systems like AAVs have limited space, often forcing you to make compromises in your design just to fit everything in. Without efficient delivery solutions, even the most carefully engineered nucleases can’t reach their full potential, stalling your progress toward therapeutic success.
The Solutions
- hfCas12Max: With a compact 1080 amino acid size, hfCas12Max easily fits into a range of delivery vehicles, including AAVs and lipid nanoparticles (LNPs). With the ability to process its own guide RNAs, hfCas12Max requires only a crRNA to act on its target, reducing the size of the necessary guide molecule and simplifying its design, and further enhancing delivery efficiency.
- SaCas9: At approximately 1kb smaller than SpCas9, SaCas9’s smaller size is perfectly suited for in vivo gene therapies that are being delivered in adeno-associated viruses (AAVs), which have a relatively small cargo-carrying capacity of around 4.5kb. SpCas9 is 4.2kb, making it impossible to fit inside the AAV along with the other required gene editing components, but SaCas9 can fit easily.
- Cas12a: Like SaCas9, Cas12a is a small nuclease and therefore easier to package inside AAV vectors for therapeutic applications. It is also able to work without a tracrRNA, making its guide molecule shorter and enabling multiplexed editing. Combined with its small size, this makes Cas12a easier to package inside AAV vectors for therapeutic applications.
- Cas12e (CasX): With a size 40% smaller than SpCas9, the PlmCas12e nuclease is capable of targeting both double-stranded and single-stranded DNA, creating staggered-end cuts in dsDNA. Even more compact in size than SaCas9 and Cas12a, this nuclease has therapeutic potential and displays robust editing in mammalian cells.
- hfCas12Max: With a compact 1080 amino acid size, hfCas12Max easily fits into a range of delivery vehicles, including AAVs and lipid nanoparticles (LNPs). With the ability to process its own guide RNAs, hfCas12Max requires only a crRNA to act on its target, reducing the size of the necessary guide molecule and simplifying its design, and further enhancing delivery efficiency.
- SaCas9: At approximately 1kb smaller than SpCas9, SaCas9’s smaller size is perfectly suited for in vivo gene therapies that are being delivered in adeno-associated viruses (AAVs), which have a relatively small cargo-carrying capacity of around 4.5kb. SpCas9 is 4.2kb, making it impossible to fit inside the AAV along with the other required gene editing components, but SaCas9 can fit easily.
- Cas12a: Like SaCas9, Cas12a is a small nuclease and therefore easier to package inside AAV vectors for therapeutic applications. It is also able to work without a tracrRNA, making its guide molecule shorter and enabling multiplexed editing. Combined with its small size, this makes Cas12a easier to package inside AAV vectors for therapeutic applications.
- Cas12e (CasX): With a size 40% smaller than SpCas9, the PlmCas12e nuclease is capable of targeting both double-stranded and single-stranded DNA, creating staggered-end cuts in dsDNA. Even more compact in size than SaCas9 and Cas12a, this nuclease has therapeutic potential and displays robust editing in mammalian cells.
Expanding CRISPR Applications to RNA Editing
Not all conditions are caused by DNA mutations. For many diseases, the problem is at the RNA level, with toxic transcripts or RNA-mediated mechanisms that disrupt cellular function. In these cases, you need RNA-editing capabilities to expand your options and develop treatments that address the root cause of these conditions.
The Solutions
- Cas13: Cas13 edits RNA instead of DNA. Like other Cas nucleases, it still works via a guide RNA, which allows it to target and cut specific cellular transcripts. It can be used for diagnostic purposes as well as the treatment of RNA-mediated diseases or conditions in which certain transcripts build up inside cells and cause toxicity. Like dCas9, Cas13 does not cause DSBs and is therefore often considered one of the potentially safer alternatives to CRISPR-Cas9.
- Cas13: Cas13 edits RNA instead of DNA. Like other Cas nucleases, it still works via a guide RNA, which allows it to target and cut specific cellular transcripts. It can be used for diagnostic purposes as well as the treatment of RNA-mediated diseases or conditions in which certain transcripts build up inside cells and cause toxicity. Like dCas9, Cas13 does not cause DSBs and is therefore often considered one of the potentially safer alternatives to CRISPR-Cas9.
Alternatives to CRISPR-Cas9 Currently in Therapeutic Development
While SpCas9 is a mainstay in the CRISPR cell and gene therapy field, many of the alternative nucleases we’ve discussed are also in various stages of clinical development. In this section, we’ll take a look at some key examples of different Cas nucleases being used in preclinical research and even some current clinical trials.
hfCas12Max treats Duchenne muscular dystrophy via exon skippingTo treat Duchenne muscular dystrophy (DMD), a fatal disorder caused by mutations in the dystrophin (DMD) gene, HuidaGene has harnessed the power of hfCas12Max to create HG302. This novel gene-editing therapy that was granted both rare pediatric disease and orphan drug designations by the FDA is delivered directly to muscle tissue by a single AAV vector. HG302 targets the splice-donor site at exon 51 of the DMD gene. By inducing exon skipping, HG302 re-establishes a correct open reading frame between exons 50 and 53, restoring expression of the dystrophin protein and improving muscle function.
To treat Duchenne muscular dystrophy (DMD), a fatal disorder caused by mutations in the dystrophin (DMD) gene, HuidaGene has harnessed the power of hfCas12Max to create HG302. This novel gene-editing therapy that was granted both rare pediatric disease and orphan drug designations by the FDA is delivered directly to muscle tissue by a single AAV vector. HG302 targets the splice-donor site at exon 51 of the DMD gene. By inducing exon skipping, HG302 re-establishes a correct open reading frame between exons 50 and 53, restoring expression of the dystrophin protein and improving muscle function.
SaCas9 therapy for the treatment of Duchenne muscular dystrophySaCas9's compact size and precise editing make it ideal for AAV-delivered gene therapies, such as those targeting DMD. By using SaCas9 and dual sgRNAs to delete multiple, problematic exons and restore the gene’s reading frame, researchers have developed a therapy that produces functional dystrophin in mouse models. The success of this approach relied on the compact size of SaCas9, which allowed both sgRNAs to fit easily within the AAV vector. This large deletion strategy, which spans a mutation hotspot, holds the potential to treat nearly 40% of DMD cases.
SaCas9's compact size and precise editing make it ideal for AAV-delivered gene therapies, such as those targeting DMD. By using SaCas9 and dual sgRNAs to delete multiple, problematic exons and restore the gene’s reading frame, researchers have developed a therapy that produces functional dystrophin in mouse models. The success of this approach relied on the compact size of SaCas9, which allowed both sgRNAs to fit easily within the AAV vector. This large deletion strategy, which spans a mutation hotspot, holds the potential to treat nearly 40% of DMD cases.
Cas12a therapies for sickle cell disease and beta-thalassemiaEditas Medicine is advancing the clinical use of Cas12a through EDIT301, an autologous ex vivo cell therapy targeting sickle cell disease (SCD) and beta-thalassemia. Instead of correcting mutations in the gene for adult hemoglobin (HbA), EDIT301 reactivates the fetal hemoglobin (Hbf) gene by inducing mutations in the HBG1/2 gamma globin promoters. This approach, used in the RUBY trial for SCD, leads to sustained Hbf production in patient-derived hematopoietic stem and progenitor cells (HSPCs), alleviating pain and reducing reliance on transfusions. With early, successful RUBY results, Editas has expanded testing to beta-thalassemia in the EdiTHAL trial.
Editas Medicine is advancing the clinical use of Cas12a through EDIT301, an autologous ex vivo cell therapy targeting sickle cell disease (SCD) and beta-thalassemia. Instead of correcting mutations in the gene for adult hemoglobin (HbA), EDIT301 reactivates the fetal hemoglobin (Hbf) gene by inducing mutations in the HBG1/2 gamma globin promoters. This approach, used in the RUBY trial for SCD, leads to sustained Hbf production in patient-derived hematopoietic stem and progenitor cells (HSPCs), alleviating pain and reducing reliance on transfusions. With early, successful RUBY results, Editas has expanded testing to beta-thalassemia in the EdiTHAL trial.
Cas3 in phage therapiesLocus Biosciences' LBP-EC01 therapy harnesses the DNA shredding ability of Cas3 within bacteriophages to target and eradicate Escherichia coli infections that cause urinary tract infections, including multi-drug resistant strains. The ELIMINATE clinical trial for LBP-EC01, currently in registration for phase II/III, offers a highly targeted approach that minimizes off-target risks and preserves beneficial bacteria. CRISPR phage (crPhage) therapy represents a groundbreaking approach and stands as the first of its kind to be tested globally, with the potential to become a leading alternative to traditional antibiotics.
Locus Biosciences' LBP-EC01 therapy harnesses the DNA shredding ability of Cas3 within bacteriophages to target and eradicate Escherichia coli infections that cause urinary tract infections, including multi-drug resistant strains. The ELIMINATE clinical trial for LBP-EC01, currently in registration for phase II/III, offers a highly targeted approach that minimizes off-target risks and preserves beneficial bacteria. CRISPR phage (crPhage) therapy represents a groundbreaking approach and stands as the first of its kind to be tested globally, with the potential to become a leading alternative to traditional antibiotics.
CasX for viral excision therapyExcision Bio has several in vivo CRISPR therapies in various stages of clinical development that are designed to cut retroviruses from the human genome using two sgRNAs. While their most advanced therapy, EBT-101, uses Cas9 to excise HIV from human cells, they are also employing CasX to tackle other viruses. Delivered via AAV, Excision’s EBT-104 therapy uses CasX with a dual sgRNA to cut herpes simplex virus (HSV) from human cells. The therapy is currently in preclinical studies. Another therapy still in proof-of-concept research is EBT-107, which uses CasX to excise the hepatitis B virus from the human genome.
Excision Bio has several in vivo CRISPR therapies in various stages of clinical development that are designed to cut retroviruses from the human genome using two sgRNAs. While their most advanced therapy, EBT-101, uses Cas9 to excise HIV from human cells, they are also employing CasX to tackle other viruses. Delivered via AAV, Excision’s EBT-104 therapy uses CasX with a dual sgRNA to cut herpes simplex virus (HSV) from human cells. The therapy is currently in preclinical studies. Another therapy still in proof-of-concept research is EBT-107, which uses CasX to excise the hepatitis B virus from the human genome.
Cas13d in Huntington’s DiseaseRNA editing using Cas13d offers a promising treatment for Huntington’s Disease (HD), a fatal neurodegenerative disorder caused by toxic protein buildup from a mutant Huntingtin gene (HTT). HDR of DSBs does not occur efficiently in neurons, and patients need the healthy HTT allele to stay intact for normal brain function, making it difficult to tackle this disease with traditional CRISPR editing. When Cas13d and an sgRNA targeting the mutant Huntingtin transcript are delivered via AAV, Cas13d is able to deplete the problematic mRNA transcripts, preventing them from being translated into toxic proteins. This method showed significant motor function improvements in HD mouse models and effectively reduced toxic proteins in patient-derived stem cells, marking a significant step towards clinical application.
RNA editing using Cas13d offers a promising treatment for Huntington’s Disease (HD), a fatal neurodegenerative disorder caused by toxic protein buildup from a mutant Huntingtin gene (HTT). HDR of DSBs does not occur efficiently in neurons, and patients need the healthy HTT allele to stay intact for normal brain function, making it difficult to tackle this disease with traditional CRISPR editing. When Cas13d and an sgRNA targeting the mutant Huntingtin transcript are delivered via AAV, Cas13d is able to deplete the problematic mRNA transcripts, preventing them from being translated into toxic proteins. This method showed significant motor function improvements in HD mouse models and effectively reduced toxic proteins in patient-derived stem cells, marking a significant step towards clinical application.
Conclusion
Engineered Cas9 variants and advancements through directed evolution are typically focused on improving CRISPR solutions for therapeutics, expanding the range and precision of gene editing capabilities. Additionally, researchers continue to mine bacterial and archaeal genomes for novel CRISPR systems, uncovering unique properties that drive innovation in both research and therapeutic development. Synthego is committed to providing you with the best of these next-generation CRISPR solutions, enabling transformative possibilities in genetic medicine.