Contents
Gene drives are self-propagating genetic elements that bias their inheritance so they can spread through populations more rapidly. Engineered gene drives have the potential to control pests or disease vectors by spreading deleterious traits, such as sterility, throughout a target population. The CRISPR revolution has accelerated the field of gene drive technology, expanding the range of applications and making it safer for deployment.
CRISPR-Cas9 technology has massively enhanced the potential of gene drive systems. CRISPR gene drives have gained significant attention for their potential to eliminate the transmission of malaria and other diseases by mosquitos. But while this is the most widely known application, it certainly isn’t the only one - CRISPR gene drives can be used to tackle a variety of real-world issues.
This blog is an updated deep dive into gene drive technology. First, we investigate what gene drives are, how they work, and the impact of the CRISPR revolution in the gene drive field, followed by an exploration of key applications of CRISPR gene drive technology. We also discuss concerns about the implementation of gene drives and strategies to address these problems.
Gene Drives: The Basics
Let’s begin our deep dive into gene drive technology with some necessary background information. In this section, we’ll explain what gene drives are, the different types of gene drives, and a brief history of their use.
What is a gene drive?As popularized in Richard Dawkin’s seminal book, The Selfish Gene, one can view natural selection acting at the level of the individual gene, rather than at the level of the organism or population. According to this viewpoint, alleles of sexually-reproducing organisms “compete” to be passed on to the next generation.
In the traditional Darwinian sense, the competition is fair— each allele has a 50% chance of being passed on to an offspring (Mendelian inheritance). Alleles that happen to benefit their hosts gradually propagate in the population while those that do not are eventually eliminated.
But what would happen if the competition was rigged and some alleles had an unfair advantage?
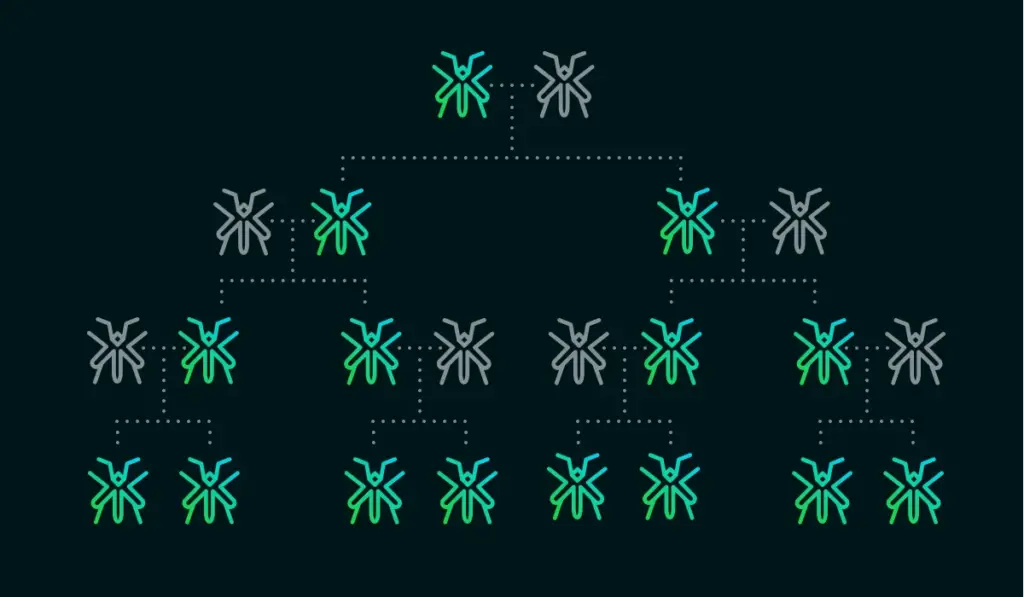
Some genes don't play by the rules. DNA sequences called gene drives ensure their own survival by biasing their transmission to an organism’s offspring. A gene drive is a self-propagating mechanism by which a desired genetic variant can be spread through a population faster than traditional Mendelian inheritance.
This strategy can be so effective that alleles can spread even if they confer a disadvantageous trait, such as sterility, to an organism. Thus, gene drives present potential new solutions for a variety of issues facing the global population, including eradicating or altering disease vectors (such as mosquitoes), controlling invasive species of plants, insects, or mammals, and combating pesticide resistance.
As popularized in Richard Dawkin’s seminal book, The Selfish Gene, one can view natural selection acting at the level of the individual gene, rather than at the level of the organism or population. According to this viewpoint, alleles of sexually-reproducing organisms “compete” to be passed on to the next generation.
In the traditional Darwinian sense, the competition is fair— each allele has a 50% chance of being passed on to an offspring (Mendelian inheritance). Alleles that happen to benefit their hosts gradually propagate in the population while those that do not are eventually eliminated.
But what would happen if the competition was rigged and some alleles had an unfair advantage?
Some genes don't play by the rules. DNA sequences called gene drives ensure their own survival by biasing their transmission to an organism’s offspring. A gene drive is a self-propagating mechanism by which a desired genetic variant can be spread through a population faster than traditional Mendelian inheritance.
This strategy can be so effective that alleles can spread even if they confer a disadvantageous trait, such as sterility, to an organism. Thus, gene drives present potential new solutions for a variety of issues facing the global population, including eradicating or altering disease vectors (such as mosquitoes), controlling invasive species of plants, insects, or mammals, and combating pesticide resistance.
Types of gene drivesThere are two main types of gene drives: natural and synthetic. As their name suggests, natural gene drives (also called selfish genetic elements) occur in nature and, as such, they can only arise through common evolutionary mechanisms.
Natural gene drives occur in a variety of organisms. For instance, transposable elements are mobile pieces of DNA that insert themselves into different parts of the genome, potentially inactivating genetic elements at their insertion sites. In fact, much of the “junk” in eukaryotic genomes originates from this type of selfish DNA.
Homing endonuclease genes (HEGs) are another type of natural gene drive found in some types of plants, fungi, and bacteria. They spread by cleaving a homologous wild-type chromosome and copying themselves into the cut site through homology-directed repair (a process called “homing”). Through this process, individuals that are heterozygous for a drive allele are turned into homozygotes. Thus, the chance of the drive allele being inherited is greater than 50%, resulting in its rapid spread throughout a population.
In contrast, synthetic gene drives are engineered in the lab to achieve desired outcomes, meaning they are not limited by natural evolutionary processes. Synthetic gene drives can be generated via various genome engineering methods, and typically must be integrated into the target organism during embryonic development before subsequent development and release into the environment.
There are two main types of gene drives: natural and synthetic. As their name suggests, natural gene drives (also called selfish genetic elements) occur in nature and, as such, they can only arise through common evolutionary mechanisms.
Natural gene drives occur in a variety of organisms. For instance, transposable elements are mobile pieces of DNA that insert themselves into different parts of the genome, potentially inactivating genetic elements at their insertion sites. In fact, much of the “junk” in eukaryotic genomes originates from this type of selfish DNA.
Homing endonuclease genes (HEGs) are another type of natural gene drive found in some types of plants, fungi, and bacteria. They spread by cleaving a homologous wild-type chromosome and copying themselves into the cut site through homology-directed repair (a process called “homing”). Through this process, individuals that are heterozygous for a drive allele are turned into homozygotes. Thus, the chance of the drive allele being inherited is greater than 50%, resulting in its rapid spread throughout a population.
In contrast, synthetic gene drives are engineered in the lab to achieve desired outcomes, meaning they are not limited by natural evolutionary processes. Synthetic gene drives can be generated via various genome engineering methods, and typically must be integrated into the target organism during embryonic development before subsequent development and release into the environment.
History of gene drive systemsThe concept of using gene drives to control the spread of pests was originally developed in the 1960s. These early ideas were based on natural gene drives that were passed on to progeny more frequently. Recent advancements in biotechnology and molecular biology methods have brought the idea of synthetic gene drives to the forefront of ecology research, and it is now an actively used strategy.
Although scientists have long recognized the potential of using gene drives to alter populations, they were previously limited by the inability to control the genomic locations that gene drives targeted and copied themselves into. However, advances in genetic engineering in the 1990s made creating endonuclease-based gene drives that could target specific locations a real possibility.
Early attempts to create endonuclease-based synthetic gene drives utilized genome engineering systems such as zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENS). Using these gene editing tools, scientists could target specific genomic sites to be altered. Although these methods enable the site-specific insertion of gene drives, they still have significant limitations. Because the sequences of these nucleases have repetitive elements, they quickly acquire mutations. The mutations often inactivate the gene drive before it can spread throughout a population.
The concept of using gene drives to control the spread of pests was originally developed in the 1960s. These early ideas were based on natural gene drives that were passed on to progeny more frequently. Recent advancements in biotechnology and molecular biology methods have brought the idea of synthetic gene drives to the forefront of ecology research, and it is now an actively used strategy.
Although scientists have long recognized the potential of using gene drives to alter populations, they were previously limited by the inability to control the genomic locations that gene drives targeted and copied themselves into. However, advances in genetic engineering in the 1990s made creating endonuclease-based gene drives that could target specific locations a real possibility.
Early attempts to create endonuclease-based synthetic gene drives utilized genome engineering systems such as zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENS). Using these gene editing tools, scientists could target specific genomic sites to be altered. Although these methods enable the site-specific insertion of gene drives, they still have significant limitations. Because the sequences of these nucleases have repetitive elements, they quickly acquire mutations. The mutations often inactivate the gene drive before it can spread throughout a population.
CRISPR Combines with Gene Drives
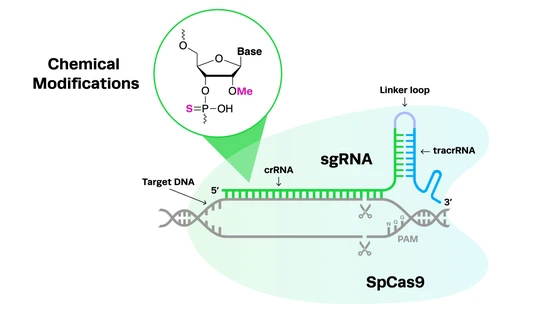
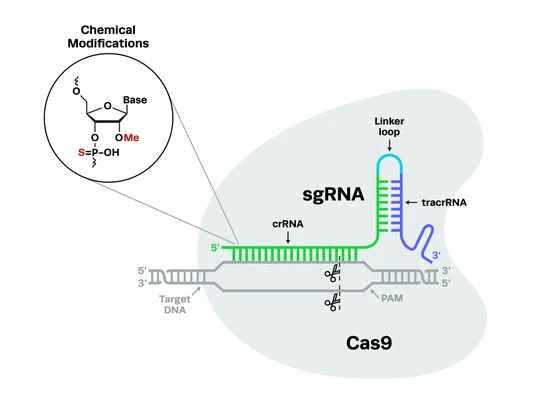
CRISPR-Cas9 technology has opened up new possibilities for developing endonuclease-based synthetic gene drives. CRISPR components can be incorporated into a “drive allele” that cuts a specific site on a wild-type homologous chromosome. In these types of gene drives, the drive allele consists of both the desired variant to be propagated and the CRISPR guide RNA and Cas9.
To repair the break, the cell uses the variant-containing chromosome as the template in a process called homology-directed repair (HDR). After the repair, the drive allele is successfully copied into the wild-type chromosome, completely replacing the wild-type DNA sequence at this position in the genome (see schematic below).
The precision, ease-of-use, and low off-target editing of CRISPR-Cas9 make it a much more attractive alternative for generating gene drives than previous genome engineering technologies. Moreover, CRISPR systems provide a wider range of genomic targets for editing and can be used to engineer organisms that have historically proven difficult to manipulate with ZFNs or TALENs.
How Does a CRISPR Gene Drive Work?
The process of engineering a gene drive for release into a wild population starts with constructing one or more transgenic organisms in the laboratory. The wild-type sequence on one chromosome is replaced with the drive allele, containing CRISPR components (guide RNA and Cas9 genes) and possibly an altered allele. The transgenic organism is then released into the wild to breed with wild-type individuals.
Two general types of gene drives can be designed, a modification drive or a suppressive drive. In a modification drive, the altered allele is spread by “hitchhiking” with the drive to wild-type chromosomes. The copying of the drive allele may occur in offspring in the early zygote stage. If this happens, all of the offspring’s cells will be homozygous for the altered gene. Alternatively, the drive may only be active in the offspring’s germline. In this case, all gametes will contain the drive allele, but cells that make up other tissues will remain heterozygous.
In suppression drives, a genetic alteration that reduces population size is spread. To achieve this, an altered allele is spread that is deleterious to the organism (e.g., causes death or infertility) when two copies are present (homozygous recessive). By copying of the drive in the germline, each offspring remains a heterozygote and thus is minimally affected by the genetic alteration. This enables the genetic alteration to spread quickly through the population at first. However, once the alteration becomes more common, homozygous offspring will be produced and the population will crash.
The altered allele generally leads to an outcome desired by the scientist that designed the drive, but is not necessarily advantageous to the organism. Some specific examples are discussed in the CRISPR gene drives applications section below.
Applications of CRISPR Gene Drives
CRISPR gene drive technology has made considerable progress in recent years. Now that we’ve discussed gene drives and how they work, let’s look at the real-world problems that can be addressed with CRISPR gene drive technology.
CRISPR mosquito gene drives eradicate malaria

One of the most common uses of gene drives is centered on eradicating vector-borne diseases - infectious diseases spread by insects or other arthropods. With changes brought about by international travel and climate change, mosquito-borne diseases are no longer confined to tropical & subtropical countries, and scientists have concluded that the best way to eradicate these diseases is to attack the mosquitos that transmit them.
Malaria affects millions of people globally every year and is caused by a Plasmodium parasite and transmitted by Anopheles gambiae mosquitoes. Several gene drive systems have been developed to control mosquito-borne diseases using gene-edited mosquitoes. In 2016, a team of researchers from Imperial College London led by Professor Andrea Crisanti developed a suppression gene drive that causes sterility in females that are homozygous recessive for the genetic alteration.
In 2018, the same group used CRISPR to modify the sex-determining gene, making the male gene dominant over the female one. The modification was then spread using a gene drive, eventually eliminating disease-transmitting female mosquitos from the population. The modified gene reached 100% prevalence within 7-11 generations, progressively reducing egg production to the point of total population collapse within the laboratory environment.
A modification gene drive that spreads a malaria-resistance gene has also been developed by scientists from John Hopkins University. Plasmodium relies on several agonists as it travels through the mosquito, and by inhibiting these agonists pre- or post-transcriptionally, transmission-blocking can be achieved.
The researchers investigated the effect of fibrinogen-related protein 1 (FREP1) gene inactivation, completely knocking out the gene using CRISPR. FREP1 knockout mosquitoes exhibited a slower pre-adult development, were less likely to feed on blood meals when given the opportunity, and laid fewer and less viable eggs when compared to their wild-type counterparts.
Gene Drive Expert Anna Buchman Discusses Controlling Insects with CRISPR
Gene drives are an application of CRISPR that hold a lot of potential. Gene drives are self-perpetuating genetic elements that bypass the rules of Mendelian genetics to quickly spread. They can be used to spread beneficial genes in the wild, or suppress populations of disease-carrying insects or agricultural pests.

Controlling other infectious disease vectors with gene drivesWhile controlling the spread of malaria in Anopheles mosquitos is perhaps the best known and most advanced application of synthetic gene drive technology, there are now a variety of other projects employing similar methods in other disease vectors.
The Aedes egypti mosquito, for example, is a vector of multiple infectious diseases that affect human populations, including yellow fever, chikungunya, dengue, and zika viruses. There has been significant research focusing on the development of gene drives in A. egypti mosquitos in order to control or prevent the spread of these diseases.
A key example is a gene drive that endows the mosquitos with antibodies against dengue fever so that they cannot contract and spread the disease. Another drive system contains a toxin that activates and kills the mosquito if it is infected with any virus. These examples of modification gene drives may allay some of the concerns surrounding the complete elimination of mosquitos from ecosystems.
While controlling the spread of malaria in Anopheles mosquitos is perhaps the best known and most advanced application of synthetic gene drive technology, there are now a variety of other projects employing similar methods in other disease vectors.
The Aedes egypti mosquito, for example, is a vector of multiple infectious diseases that affect human populations, including yellow fever, chikungunya, dengue, and zika viruses. There has been significant research focusing on the development of gene drives in A. egypti mosquitos in order to control or prevent the spread of these diseases.
A key example is a gene drive that endows the mosquitos with antibodies against dengue fever so that they cannot contract and spread the disease. Another drive system contains a toxin that activates and kills the mosquito if it is infected with any virus. These examples of modification gene drives may allay some of the concerns surrounding the complete elimination of mosquitos from ecosystems.
Using CRISPR gene drives to target vectors and reservoirs of Lyme diseaseGene drive technology also has the potential to control the transmission of Lyme disease, a debilitating illness which is increasing in prevalence. In the US, white-footed mice are the main reservoir of the Borrelia bacteria that causes the disease, and this bacteria is transmitted from mice to humans through the bites of black-legged ticks. Modification or suppression gene drives could be used to alter populations of black-legged ticks, preventing transmission of the disease.
Gene editing progress in ticks has been slow, largely because ticks coat their eggs in large amounts of wax, preventing the successful injection of CRISPR components into embryos at the correct developmental stage without destroying the egg in the process. However, research published in early 2022 described a novel protocol for CRISPR-based editing of tick embryos, providing a breakthrough that may enable the development of gene drives in ticks.
Kevin Esvelt, an early CRISPR pioneer who leads a group of researchers at MIT, is also working towards tackling Lyme disease with CRISPR technology. Rather than targeting the disease vector, Esvelt and his team are using CRISPR to make the reservoir of white-footed mice immune to the bacteria. In a project entitled “Mice Against Ticks,” Esvelt and his team are working with the communities of Martha’s Vineyard and Nantucket to use CRISPR to eradicate the disease.
The “Mice Against Ticks” project does not involve a gene drive, but rather the natural propagation of edited alleles through a population. Esvelt and his team have proposed using CRISPR to introduce an allele into some mice that immunizes them against the disease. The genetically modified mice would then be released so they can breed with wild mice. In this way the allele can naturally propagate through the wild mouse populations on the islands.
The potential release of genetically-modified mice is years away and must be approved by state and federal regulators, each island's community-led Steering Committee, an independent group of scientists and ultimately at the ballot box. Through transparency and public involvement, Esvelt has demonstrated a more open way of doing science.
Gene drive technology also has the potential to control the transmission of Lyme disease, a debilitating illness which is increasing in prevalence. In the US, white-footed mice are the main reservoir of the Borrelia bacteria that causes the disease, and this bacteria is transmitted from mice to humans through the bites of black-legged ticks. Modification or suppression gene drives could be used to alter populations of black-legged ticks, preventing transmission of the disease.
Gene editing progress in ticks has been slow, largely because ticks coat their eggs in large amounts of wax, preventing the successful injection of CRISPR components into embryos at the correct developmental stage without destroying the egg in the process. However, research published in early 2022 described a novel protocol for CRISPR-based editing of tick embryos, providing a breakthrough that may enable the development of gene drives in ticks.
Kevin Esvelt, an early CRISPR pioneer who leads a group of researchers at MIT, is also working towards tackling Lyme disease with CRISPR technology. Rather than targeting the disease vector, Esvelt and his team are using CRISPR to make the reservoir of white-footed mice immune to the bacteria. In a project entitled “Mice Against Ticks,” Esvelt and his team are working with the communities of Martha’s Vineyard and Nantucket to use CRISPR to eradicate the disease.
The “Mice Against Ticks” project does not involve a gene drive, but rather the natural propagation of edited alleles through a population. Esvelt and his team have proposed using CRISPR to introduce an allele into some mice that immunizes them against the disease. The genetically modified mice would then be released so they can breed with wild mice. In this way the allele can naturally propagate through the wild mouse populations on the islands.
The potential release of genetically-modified mice is years away and must be approved by state and federal regulators, each island's community-led Steering Committee, an independent group of scientists and ultimately at the ballot box. Through transparency and public involvement, Esvelt has demonstrated a more open way of doing science.
Gene drives in pathogens: targeting species without sexual reproductionRather than targeting vectors of disease, such as mosquitos, CRISPR-Cas9 gene drives can also be used to target the pathogens themselves. Due to the premise of gene drives being spread through sexual reproduction, it was widely assumed that this technology could not be adapted for pathogens - such as viruses and bacteria - that do not reproduce sexually.
In 2020, however, a proof-of-principle study published in Nature Communications demonstrated that CRISPR gene drives can be successfully transmitted between different strains of human herpesviruses, which cause many human diseases. This strategy relies on the co-infection of cells by both wild-type and drive-containing viruses; herpesviruses frequently undergo homologous recombination in their double-stranded DNA genomes, which allows for the alteration of the wildtype viruses by the engineered variants within the same cell.
In these breakthrough experiments, the viruses containing the drive were shown to replace wild-type populations in cell culture, with the engineered viruses displaying significantly reduced infectivity. These results prove that gene drives can work in viruses and offer a method to suppress viral infections because the drive targeted viral replication genes.
Gene drive technology has also been explored in other non-sexually reproducing pathogens - for instance, they have been used to inactivate an antibiotic resistance marker in Escherichia coli. CRISPR gene drives are also being explored in fungi, including the opportunistic pathogen Candida albicans.
Rather than targeting vectors of disease, such as mosquitos, CRISPR-Cas9 gene drives can also be used to target the pathogens themselves. Due to the premise of gene drives being spread through sexual reproduction, it was widely assumed that this technology could not be adapted for pathogens - such as viruses and bacteria - that do not reproduce sexually.
In 2020, however, a proof-of-principle study published in Nature Communications demonstrated that CRISPR gene drives can be successfully transmitted between different strains of human herpesviruses, which cause many human diseases. This strategy relies on the co-infection of cells by both wild-type and drive-containing viruses; herpesviruses frequently undergo homologous recombination in their double-stranded DNA genomes, which allows for the alteration of the wildtype viruses by the engineered variants within the same cell.
In these breakthrough experiments, the viruses containing the drive were shown to replace wild-type populations in cell culture, with the engineered viruses displaying significantly reduced infectivity. These results prove that gene drives can work in viruses and offer a method to suppress viral infections because the drive targeted viral replication genes.
Gene drive technology has also been explored in other non-sexually reproducing pathogens - for instance, they have been used to inactivate an antibiotic resistance marker in Escherichia coli. CRISPR gene drives are also being explored in fungi, including the opportunistic pathogen Candida albicans.
CRISPR in Fungi: An Interview with Rebecca Shapiro
Rebecca Shapiro, Assistant Professor at the University of Guelph, talks about CRISPR genome editing in fungal pathogens to understand their disease-causing mechanism. Her lab also works on gene drives in fungi for studying large scale genetic interactions and developing ways for treating fungal biofilms.

Controlling invasive species & pests with gene drivesSimilar to eradicating unwanted diseases, CRISPR can be used to develop a gene drive to control or eliminate invasive species. Species that are introduced to areas where they do not occur naturally can do a substantial amount of ecological and economical damage. They may compete with or prey on native species, sometimes driving them to extinction. Suppression or modification gene drives can be used to eradicate species in areas where they are not native.
One study used computer models to assess CRISPR-based gene drives that could potentially eradicate invasive vertebrates, like mice, rats, and rabbits, from islands by causing death or sterility in homozygous females or changing females into sterile males. The Genetic Control of Invasive Rodents (GBIRd) program, made up of university, government, and non-profit agency partners, is dedicated to advancing gene drive research in order to eliminate pest rodents.
Gene drives can also be used to reduce agricultural pests' population size. Currently, farms often use pesticides to keep crop-eating pests at bay. However, the pest populations often evolve resistance to pesticides over time. A CRISPR-based drive could be used to spread a genetic alteration that re-sensitizes pests to toxins, or makes them sensitive to otherwise innocuous compounds.
Early in 2022, researchers from UC San Diego published a Nature Communications study describing a gene drive system in fruit flies to reverse insecticide resistance. The group targeted the voltage-gated sodium channel (VGSC) protein, replacing an allele that encodes insecticide resistance in the pest flies with an insecticide-susceptible variant. The authors postulate that this method has the potential to greatly reduce the amount of pesticides used in agriculture and suggest that it could also be applied to the malaria mosquito problem.
Similar gene drives can be utilized to render common weed plants susceptible to herbicides, while the reverse can be applied to crop plants in order to generate varieties that are resistant to widely-used herbicides.
Similar to eradicating unwanted diseases, CRISPR can be used to develop a gene drive to control or eliminate invasive species. Species that are introduced to areas where they do not occur naturally can do a substantial amount of ecological and economical damage. They may compete with or prey on native species, sometimes driving them to extinction. Suppression or modification gene drives can be used to eradicate species in areas where they are not native.
One study used computer models to assess CRISPR-based gene drives that could potentially eradicate invasive vertebrates, like mice, rats, and rabbits, from islands by causing death or sterility in homozygous females or changing females into sterile males. The Genetic Control of Invasive Rodents (GBIRd) program, made up of university, government, and non-profit agency partners, is dedicated to advancing gene drive research in order to eliminate pest rodents.
Gene drives can also be used to reduce agricultural pests' population size. Currently, farms often use pesticides to keep crop-eating pests at bay. However, the pest populations often evolve resistance to pesticides over time. A CRISPR-based drive could be used to spread a genetic alteration that re-sensitizes pests to toxins, or makes them sensitive to otherwise innocuous compounds.
Early in 2022, researchers from UC San Diego published a Nature Communications study describing a gene drive system in fruit flies to reverse insecticide resistance. The group targeted the voltage-gated sodium channel (VGSC) protein, replacing an allele that encodes insecticide resistance in the pest flies with an insecticide-susceptible variant. The authors postulate that this method has the potential to greatly reduce the amount of pesticides used in agriculture and suggest that it could also be applied to the malaria mosquito problem.
Similar gene drives can be utilized to render common weed plants susceptible to herbicides, while the reverse can be applied to crop plants in order to generate varieties that are resistant to widely-used herbicides.
Adapting gene drives to protect endangered species

While gene drives are most commonly used to control or decrease the population of target organisms, they may also be used to help save species that are in danger of becoming extinct. Through modification gene drives, a protective gene could be spread. For instance, frogs and other amphibians are in dramatic decline worldwide, largely due to pathogenic chytrid fungus. The fungus causes a skin disease that is often lethal. A gene preventing fungal infections could potentially save many frogs and other amphibians from extinction.
The idea of CRISPR gene drives has also been suggested for the conservation of endangered plant species, particularly as the effects of climate change continue to strain and restrict wild populations. Adding useful genes to endangered plants, such as drought tolerance or disease-resistance genes, can potentially ensure their long-term survival.
Responsible Implementation of Gene Drive Technology: Safety, Ethics, and Effectiveness
The CRISPR gene drive field is really only in its infancy, and it can be controversial. Let’s explore some key concepts surrounding the responsible use of CRISPR gene drives, including effectiveness, resistance, safety, and control methods.
Effectiveness of CRISPR-based gene drives and drive resistanceThere are several factors that can affect the efficacy of CRISPR-based gene drives. Drive alleles that are stable over time will spread to more individuals, while those that accumulate mutations can become resistant to the drive mechanism. The fitness cost of a drive allele can also affect how much it spreads. For instance, an allele that substantially decreases the fitness in heterozygous individuals will not spread as effectively as alleles that have a minimal fitness cost.
Additionally, copying of the drive allele into wild-type chromosomes will only occur if the cell uses homology-directed repair to mend the double-strand break. The cell may instead repair the break via non-homologous end joining repair (NHEJ). This pathway pastes the ends together without using a repair template and therefore does not copy the drive gene into a wild-type chromosome. Thus, the frequencies of HDR relative to NHEJ will affect the efficiency of a gene drive. Several characteristics of the target organism can also affect gene drives, including the time it takes to produce a new generation and mating frequency.
There are several efforts to overcome resistance to CRISPR gene drives and safeguard their spread. For example, one strategy is touse multiplexed sgRNAs to increase the effective homing rate and reduce the generation of resistant alleles. The other is to target highly conserved regions.
There are several factors that can affect the efficacy of CRISPR-based gene drives. Drive alleles that are stable over time will spread to more individuals, while those that accumulate mutations can become resistant to the drive mechanism. The fitness cost of a drive allele can also affect how much it spreads. For instance, an allele that substantially decreases the fitness in heterozygous individuals will not spread as effectively as alleles that have a minimal fitness cost.
Additionally, copying of the drive allele into wild-type chromosomes will only occur if the cell uses homology-directed repair to mend the double-strand break. The cell may instead repair the break via non-homologous end joining repair (NHEJ). This pathway pastes the ends together without using a repair template and therefore does not copy the drive gene into a wild-type chromosome. Thus, the frequencies of HDR relative to NHEJ will affect the efficiency of a gene drive. Several characteristics of the target organism can also affect gene drives, including the time it takes to produce a new generation and mating frequency.
There are several efforts to overcome resistance to CRISPR gene drives and safeguard their spread. For example, one strategy is touse multiplexed sgRNAs to increase the effective homing rate and reduce the generation of resistant alleles. The other is to target highly conserved regions.
Safety and ethical issues associated with CRISPR gene drivesAlthough CRISPR-based gene drives have the potential to solve a lot of problems, many scientists and members of the public have voiced valid concerns over their use. In fact, many have concerns about whether humans should alter natural ecosystems at all. Releasing any genetically-modified organism into wild populations must be done responsibly, carefully considering potential ecological impacts.
For instance, a gene drive that would eradicate an entire mosquito population in a given area may disrupt the ecosystem in unforeseen ways. While an alternative is to render the disease vector immune to the pathogen, there are also concerns surrounding the impact of this change; by eliminating one pathogen, does this simply provide an empty niche for other - potentially worse - pathogens to evolve?
Pilot projects of gene drives in wild populations have been confined to island populations that cannot easily escape. However, if the technology were to be used on larger continents, there would be no easy method to prevent organisms containing gene drives from crossing country borders.
Although CRISPR-based gene drives have the potential to solve a lot of problems, many scientists and members of the public have voiced valid concerns over their use. In fact, many have concerns about whether humans should alter natural ecosystems at all. Releasing any genetically-modified organism into wild populations must be done responsibly, carefully considering potential ecological impacts.
For instance, a gene drive that would eradicate an entire mosquito population in a given area may disrupt the ecosystem in unforeseen ways. While an alternative is to render the disease vector immune to the pathogen, there are also concerns surrounding the impact of this change; by eliminating one pathogen, does this simply provide an empty niche for other - potentially worse - pathogens to evolve?
Pilot projects of gene drives in wild populations have been confined to island populations that cannot easily escape. However, if the technology were to be used on larger continents, there would be no easy method to prevent organisms containing gene drives from crossing country borders.
Control and reversal of CRISPR gene drivesDue to the potential negative outcomes and ethical issues associated with the use of gene drives in wild populations, there has been a significant amount of research focusing on the control and reversal of gene drives. A key example is the development of confinable split gene drives, in which Cas9 is inserted at one location in the genome, while the other genetic element/s are inserted to a different location. These drives have been suggested to be safer and more suitable for release.
Self-exhausting, ‘daisy-chain’ drives have also been developed with CRISPR technology. These drives are fast-acting but transient, with elements successively lost over time to control the spread, meaning they do not indefinitely drive an allele.
Due to the potential negative outcomes and ethical issues associated with the use of gene drives in wild populations, there has been a significant amount of research focusing on the control and reversal of gene drives. A key example is the development of confinable split gene drives, in which Cas9 is inserted at one location in the genome, while the other genetic element/s are inserted to a different location. These drives have been suggested to be safer and more suitable for release.
Self-exhausting, ‘daisy-chain’ drives have also been developed with CRISPR technology. These drives are fast-acting but transient, with elements successively lost over time to control the spread, meaning they do not indefinitely drive an allele.
CRISPR Gene Drives Promise a Bright Future
The global pandemic of the SARS-CoV-2 virus, which causes COVID-19, has piqued interest in using gene drives to prevent the transmission of deadly pathogens from animals to humans. For example, some researchers have recently suggested that engineering gene drives in bats could prevent future pandemics. To prevent the spillover of viral pathogens from bats to humans, gene drives could theoretically be engineered to render wild bats immune to coronaviruses, such as those that have caused the SARS, MERS, and COVID-19 pandemics.
Despite its promise, much work still needs to be done to ensure CRISPR gene drives are used safely in wild populations. Going forward, it will be important for all researchers to utilize gene drives with a sense of awareness and caution. Gene drives have the potential to become one of the most impactful advancements in human history, but this potential will only be realized through careful and responsible use.
Want more CRISPR? Check out this list of our favorite CRISPR podcasts to keep learning, or subscribe to The Bench Blog for regular updates on CRISPR technology and its applications. If you’re keen to use CRISPR in your research, let's chat more to see how we can help you.