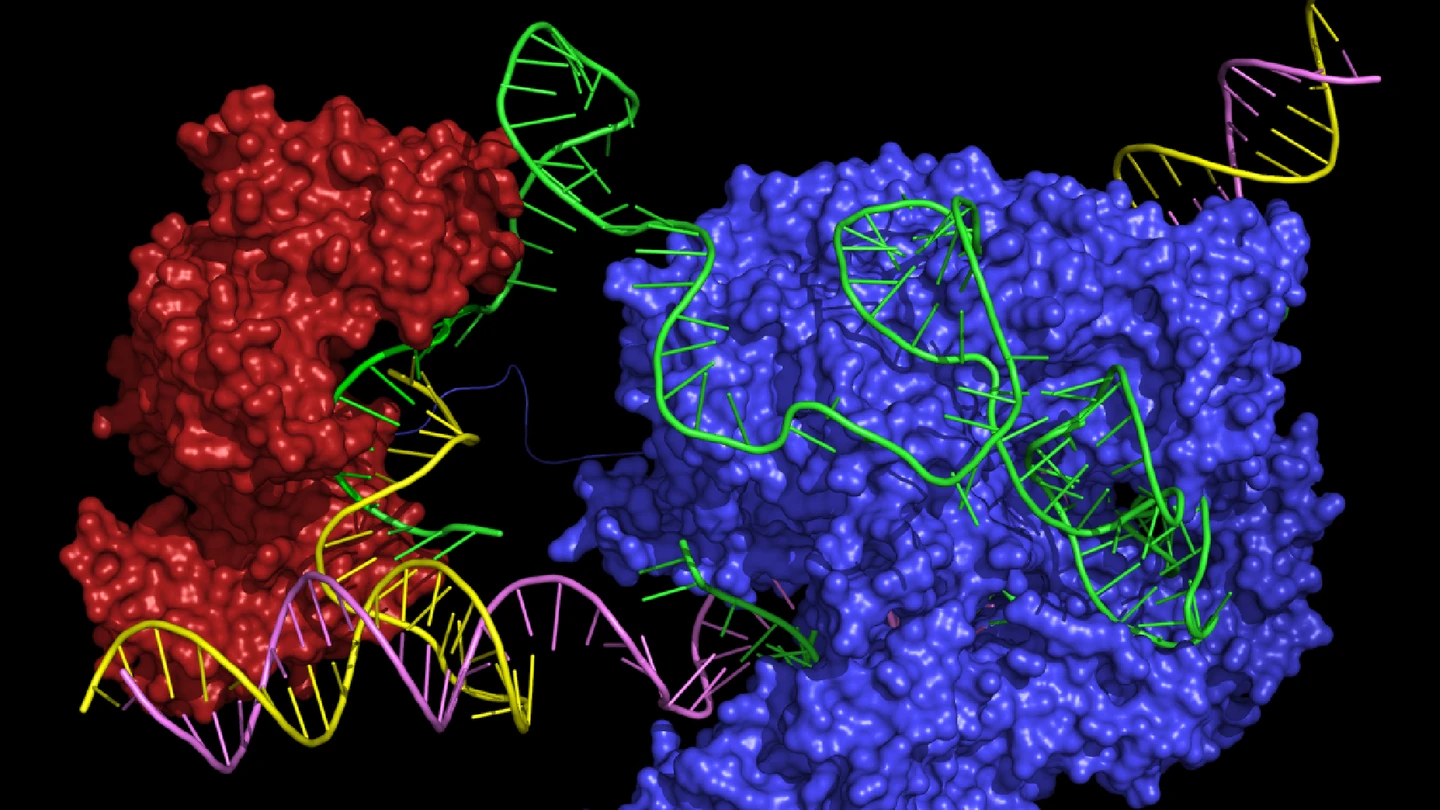
Base editing relies on the fusion of a deaminase enzyme with a catalytically impaired variant of a Cas nuclease, typically Cas9 or Cas12. The catalytically impaired Cas protein is engineered to retain its DNA-binding but is rendered incapable of introducing double-strand breaks. Instead, it precisely positions the deaminase to act on a target base within a specific DNA sequence. The Cas protein’s function in this system is purely to facilitate sequence targeting and spatial positioning, ensuring that the deaminase can act with high precision at the intended site within the genome.
Dead Cas9 (dCas9) and Cas9 nickase (nCas9) are widely used in base editing. dCas9 is a catalytically inactive Cas9 variant that binds DNA with high precision, guided by a base editing gRNA molecule, but lacks nuclease activity. This allows it to act solely as a DNA-binding scaffold, positioning the fused deaminase enzyme directly at the target site. Cas9 nickase, on the other hand, retains one active nuclease domain, introducing a single-strand nick instead of a cut through both strands. While this nick can enhance the DNA repair process, increasing the efficiency of the intended base conversion, it can also result in unintended insertions or deletions (indels) at the repair site, which may compromise editing precision. Both variants, however, remain crucial components of base editors for achieving precise and controlled genetic modifications.