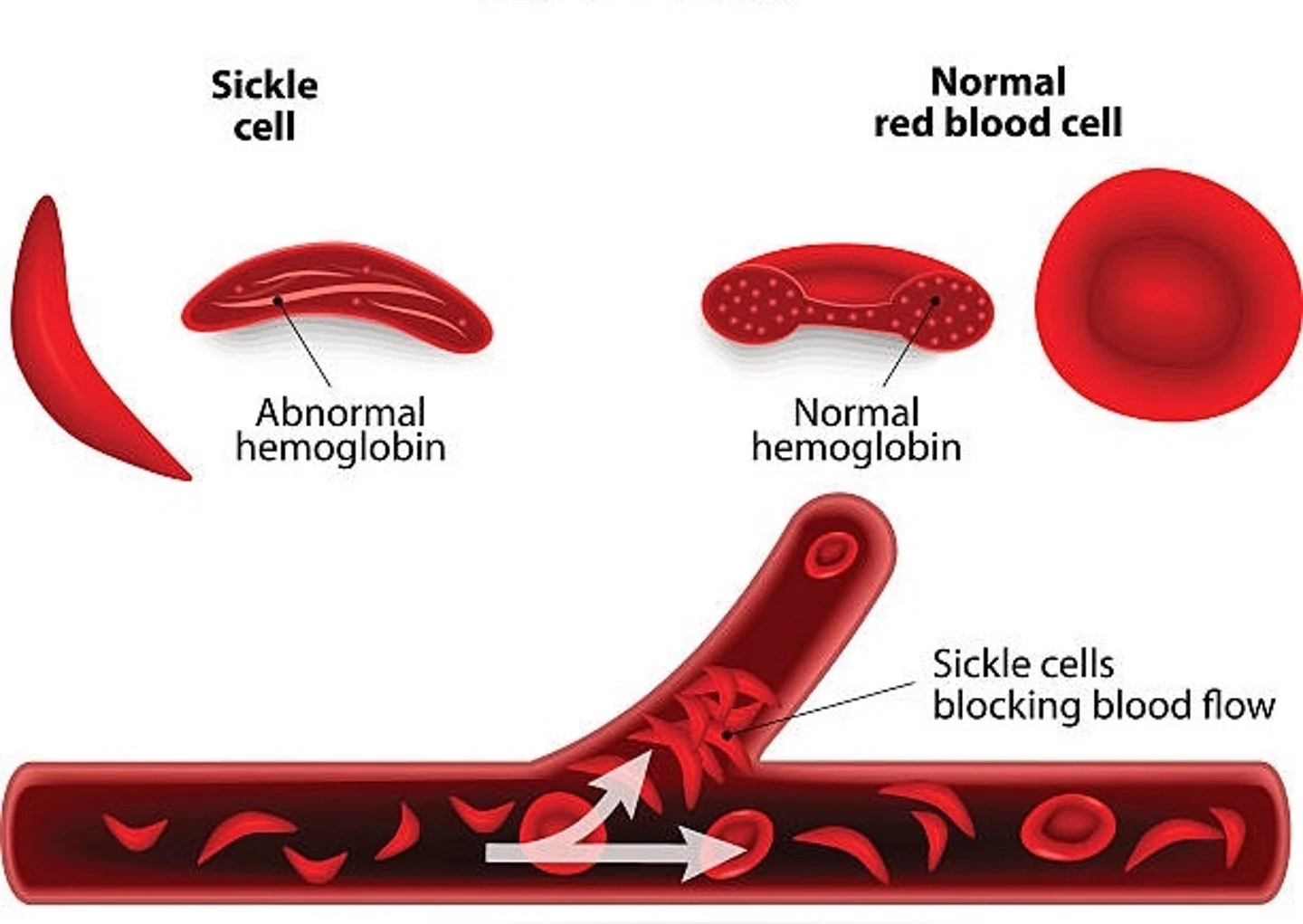
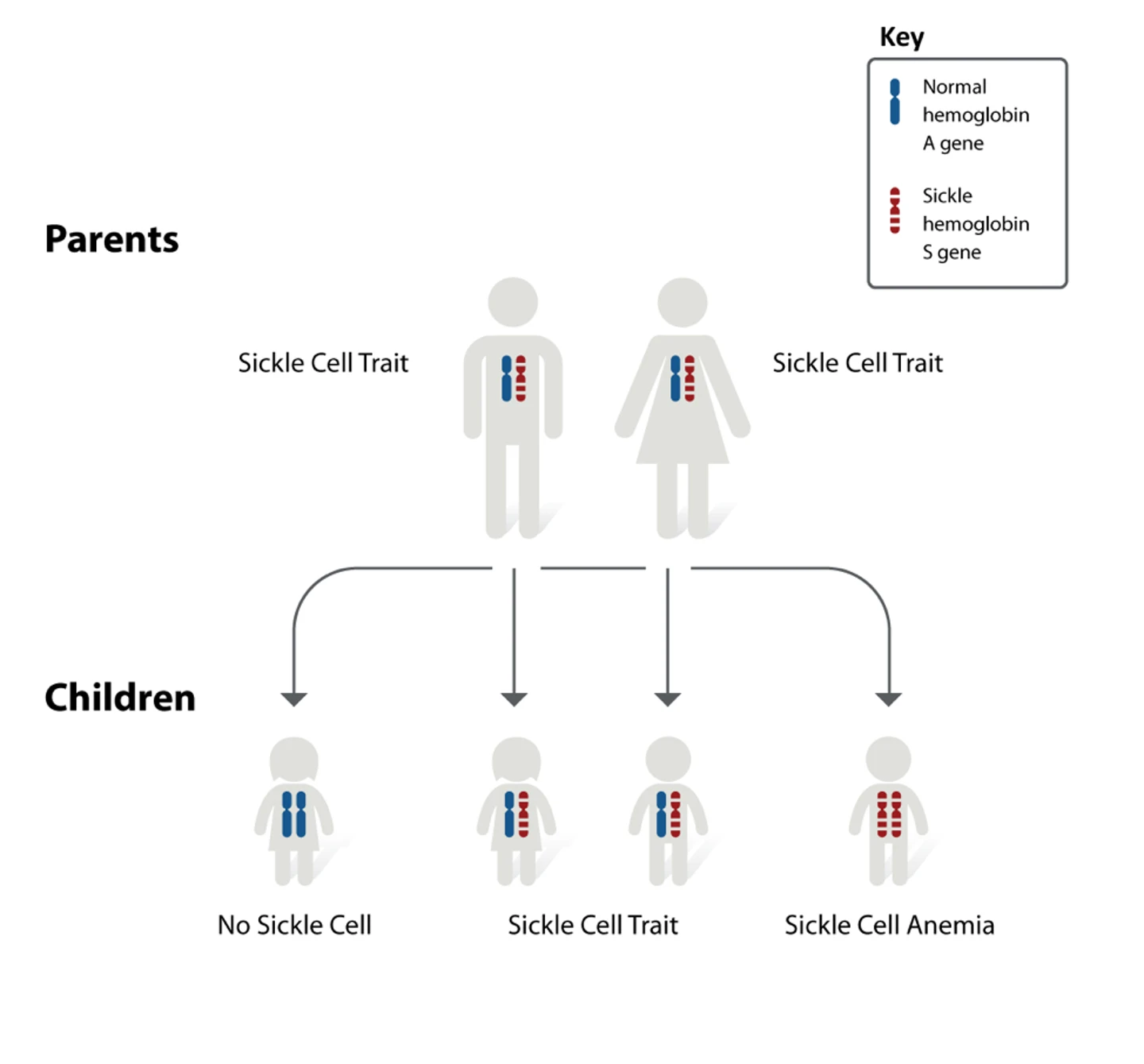
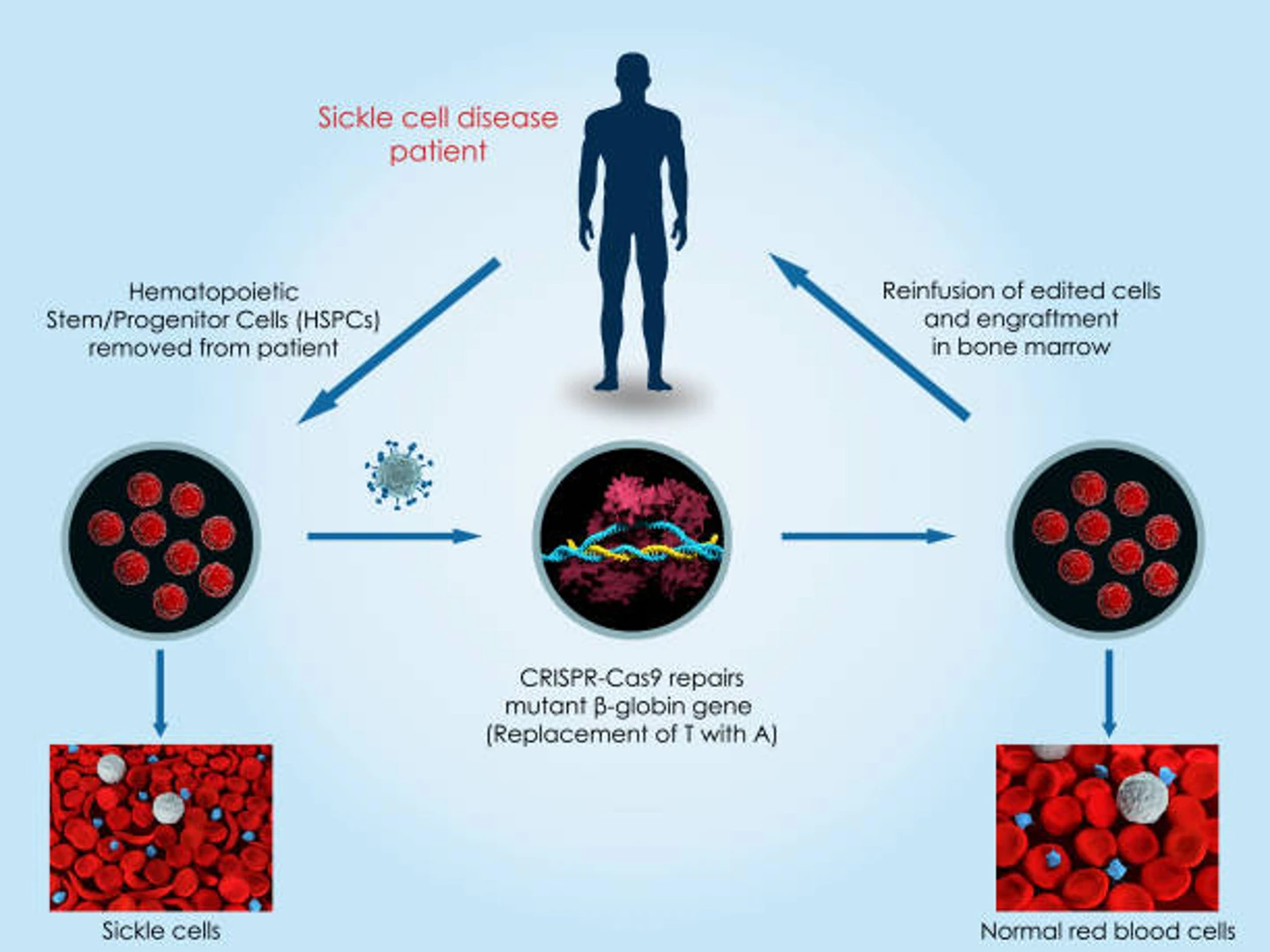
In 2021, the, the FDA has approved a new clinical trial to treat the root cause of sickle cell anemia. Spearheaded by Dr. Mark Walters, professor of pediatrics at UCSF, the autologous cell therapy trial aims to directly target and correct the disease-causing mutation via gene knock-in. The researchers predict that editing efficiencies of 20% should be enough to result in significant clinical benefit to sickle cell patients.
Beam Therapeutics has also set its sights on treating sickle cell disease via a base editing approach and received FDA clearance to begin clinical trials. The treatment, known as BEAM-101, is an autologous hematopoietic cell therapy that introduces point mutations known to occur in people who maintain the expression of fetal hemoglobin. Beam Therapeutics is also in the process of gathering preclinical data for another base editing treatment, BEAM-102, which corrects the disease-causing mutation in the adult hemoglobin gene. In 2022, BEAM Therapeutics submitted their IND for BEAM-102.
As current CRISPR-based therapies progress through the clinic and new therapies are developed, assessing their safety and efficacy is crucial as this groundbreaking technology continues to revolutionize the landscape of medical treatment. Rigorous evaluation ensures that these innovative approaches do not pose unintended risks while maximizing their therapeutic potential. As CRISPR offers unprecedented precision in gene editing, it is reshaping how therapies are developed, enabling scientists to target and potentially cure genetic disorders with remarkable accuracy.
Furthermore, scientists are actively exploring novel nucleases as alternatives because they lower the entry barrier to developing therapies because of their improved licensing structures and gene editing versatility. These novel nucleases, including hfCas12Max and eSpOT-ON (as recombinant protein or mRNA), offer distinct advantages in terms of target specificity and reduced off-target effects, broadening the range of applications for CRISPR-based therapies. By integrating these alternative nucleases into their toolkit, scientists can refine existing therapies and develop innovative solutions for previously challenging gene targets. This evolution in the CRISPR landscape not only accelerates the therapeutic development process but also fosters personalized medicine, tailoring treatments to individual genetic profiles. As we move forward, ongoing research and regulatory scrutiny will be essential to harnessing the full potential of CRISPR, paving the way for a future where previously untreatable diseases may become manageable or even curable.