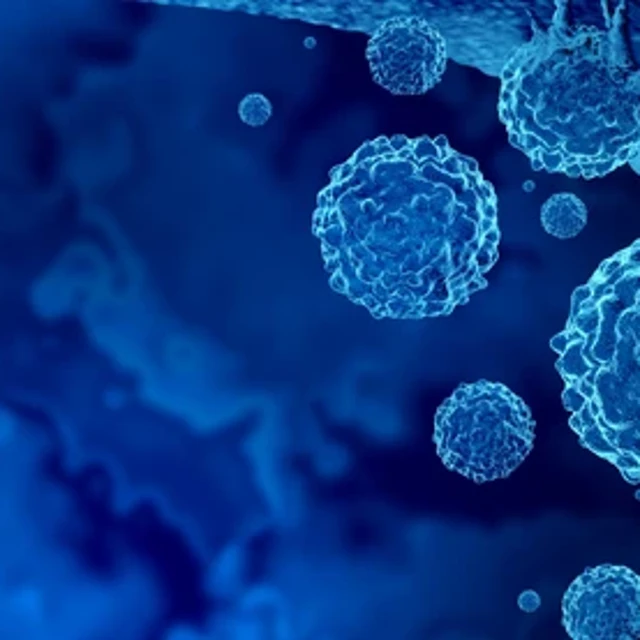
"We are seeing a cancer drug being made from a person's own cells that did not cause disease in that person before, and did not cause toxicity in that person before. So, if our modifications don't go on to cause toxicity—and that's something that the field definitely has to work on and be very conscious about—then we are actually improving on the safety profile of cancer therapies by using immunotherapies."Avery Posey, leading CAR-T cell researcher at the University of Pennsylvania.

CRISPR in Cancer Therapy eBook
Cancer affects millions worldwide, leading to deaths and treatment-related adverse health effects while significantly burdening the economy. Conventional therapies may seem adequate for a while but eventually fail patients. This treatment failure is primarily due to increased drug resistance.
The genetic basis for cancer means that it can be targeted with genome editing technologies, such as CRISPR. This explains why CRISPR genome engineering is creating a revolution in cancer therapy.
Read our eBook to stay updated on the latest trends in the field.