Cell-based therapies have been in development for many years, but as one might suspect, it’s much more difficult than it sounds. While the incredible breakthroughs in gene editing enabled by CRISPR have helped scientists edit cells easier and more efficiently than ever before, translating CRISPR into the clinic to provide meaningful therapies still has a way to go due to concerns over cost, safety, and efficacy. Researchers at UC San Francisco have recently discovered a new way to engineer immune system cells in a safer and more effective manner to better understand and begin to treat a variety of diseases such as common cancers to rare genetic disorders.
In the July 11, 2018 issue of Nature, Theodore Roth and colleagues in the Marson lab at UCSF published a report in which they used CRISPR-Cas9 to edit a specific type of cell in the immune system, the T cell, using a method that is safer and more effective than previous approaches. They used this method to successfully correct a genetic mutation in T cells and restore their function.
Additionally, they were able to edit receptors on T cells that allow them to recognize and eliminate cancer cells within the body. This monumental advancement brings sc
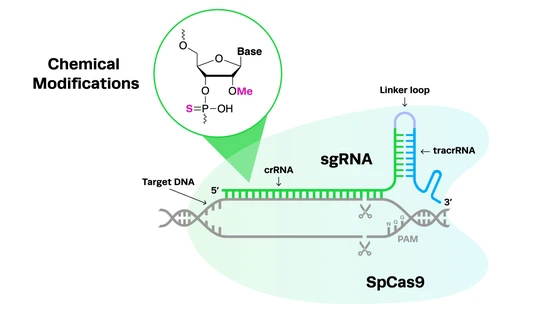
Editing T Cells Using CRISPR-Cas9
Traditional genome editing strategies have previously used viruses to introduce RNA or DNA into cells, which has several disadvantages. Viruses can randomly integrate their genome sequences into the genome of the cell being edited, which causes additional unwanted DNA edits. Most viruses also have limitations on the size of the DNA that they can deliver into cells (longer DNA sequences that are required to edit T cell genomes for therapeutic use are difficult to incorporate into the commonly-used viral vectors). Additionally, virus production and quality control are expensive and time-consuming and, in some cases, viruses can be toxic to cells.
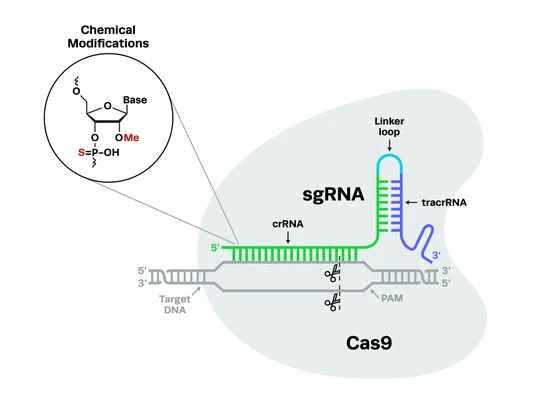
Roth and colleagues developed a new way to edit T cells by using CRISPR-Cas9 without using viruses. In this system, the researchers use a method called electroporation, which uses electricity to generate holes in the cell membrane that allow the CRISPR components to enter. Synthetic guide RNAs (gRNAs) complexed to the Cas9 protein, along with the DNA donor template used to create CRISPR knock-ins, enter the cell through these holes and go to the targeted spot on the genome to perform the editing. Cas9 then cuts the DNA and the cell’s internal repair mechanisms take over to rejoin the DNA at the break site.
To investigate the specificity of this method, Roth et al. performed a knock-in experiment and tagged several T cell proteins with green fluorescent protein (GFP). They found that GFP appeared where expected within the cell, with very low evidence of any non-specific editing. Along with confirming specificity, they also optimized the procedure to generate both high immune cell viability and high CRISPR editing efficiency.
CRISPR Editing for Cell-Based Therapies to Cure Autoimmune Diseases
Editing genes in cells is one thing; being able to use those edited cells to benefit patients is a whole other story. Roth and colleagues extended the findings of their virus-free synthetic gRNA CRISPR editing method in two clinically relevant scenarios.
In one experiment, they tested whether they could correct a genetic mutation in T cells that causes them to be non-functional and leads to an autoimmune disorder. When they corrected the genetic sequence, they saw restoration of gene expression and functional signaling, demonstrating that this virus-free CRISPR editing approach can be an effective therapeutic strategy for correcting point mutations in patients.
These results are promising and provide hope for being able to use CRISPR to correct mutations that lead to diseases in patients. This type of therapy would allow doctors to repair the cause of the disease, rather than trying to treat the symptoms, which would completely revolutionize medicine.
Moving CRISPR Into the Clinic: Virus-Free CAR T Therapy to Fight Cancer
Next, they wanted to test whether their non-viral synthetic gRNA-based CRISPR gene engineering method could be used to insert large DNA sequences into the genome, specifically regarding CAR T therapy.
CAR T therapy relies on the editing of T cells to express a chimeric antigen receptor (CAR) on the cell’s surface. The CAR recognizes antigens specific to types of cancer cells. Therefore, the engineered CAR T cell can circulate and recognize cancer cells, bind to those cell’s antigen and eliminate the cells from the body.
While evidence supporting the clinical use CAR T therapy is still growing, initial success stories in a small set of clinical trials resulted in two CAR T therapies being approved by the FDA in 2017, and we expect additional progress in 2018.
Despite these advances, CAR T therapies have previously relied on the use of viruses, which is not ideal for clinical use. Roth and colleagues edited the CAR receptors on T cells by inserting a 1.5 kb DNA sequence in the T cell receptor gene using their synthetic guide RNA CRISPR-Cas9 method. Following CRISPR editing, the modified T cells responded to the presence of cancer cells by producing cytokines, and more importantly, they killed cancer cells at a rate similar to virally-edited T cells.
This study marks the first ever reported method of performing CAR T therapy in a virus-free manner.
The successful editing of T cells without using viruses is a breakthrough not only in immunotherapy, it also opens the door to adapt this method for other cell-based therapies. Using synthetic gRNA for CRISPR editing takes a shorter amount of time than the previous virus-based methods, is much safer, and is just as effective as viral editing strategies, making it more suitable to use in the clinic.
Safe and effective genome engineering of patient’s immune cells can lead to novel therapies to improve their lives.