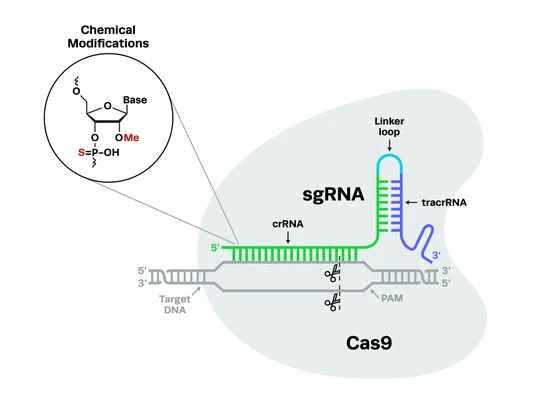
The advent of novel gene-editing tools like CRISPR-Cas9 has made it easier to genetically edit animal models. The choice of a model organism to study human diseases is typically a trade-off between how simple it to manipulate the organism and how closely the model mimics the human disease.
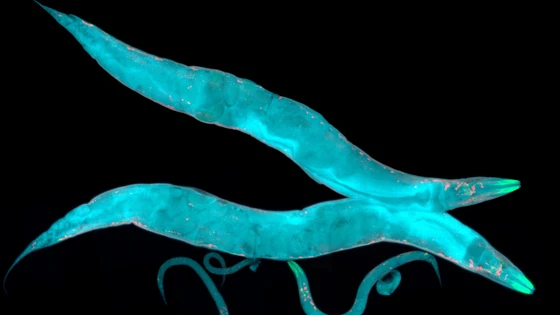
In this regard, Caenorhabditis elegans (C. elegans), a microscopic, soil-dwelling nematode worm, with a short lifespan of 2-3 weeks, has been a popular choice for the study of the development, signaling pathways, and general biological processes.
Several advances have been made in human disease genetics using C. elegans as a non-mammalian model organism, which have been further accelerated by the introduction of CRISPR technology. In this article, we will learn about C. elegans, its genetics, and how it is playing an important role as a model organism in CRISPR research. Specifically, we will cover the following topics:
What is C. elegans and Why is it Important for Research?
Caenorhabditis elegans (C. elegans) is a non-pathogenic, microscopic nematode roundworm that has been used as a model organism in research since the 1960s. This tiny organism is about 1 mm in length, lives in soil, and feeds on microbes. It was initially used as a model organism for developmental biology but has now gained popularity for modeling many human diseases, such as neurological disorders, kidney and heart disease, diabetes, and cancer.
C. elegans: Life cycle and developmentC. elegans is conceived as a single cell and it undergoes embryonic cleavage, morphogenesis, and growth to the adult phase. These worms are transparent and have a short life cycle of 2-3 weeks which makes it easy to study their development. Although these tiny worms are much simpler than humans and do not have skeletal or circulatory systems, they do share many genes and molecular pathways with us. Hence C. elegans offers researchers an ideal compromise between tractability and complexity.
C. elegans is conceived as a single cell and it undergoes embryonic cleavage, morphogenesis, and growth to the adult phase. These worms are transparent and have a short life cycle of 2-3 weeks which makes it easy to study their development. Although these tiny worms are much simpler than humans and do not have skeletal or circulatory systems, they do share many genes and molecular pathways with us. Hence C. elegans offers researchers an ideal compromise between tractability and complexity.
C. elegans genome: Size & sequencingThe complete genome sequence of C. elegans was published in 1998. This nematode worm was the first multicellular organism to have its whole genome sequenced. Its genome is about 100 million base pairs in length and contains about 20,000 protein coding genes, which is a similar number of genes as humans.
Many genes known to be associated with human disease have a counterpart in C. elegans, making it a very useful model for disease research. C. elegans mutants have provided models for many neurological, cardiac and kidney diseases in humans. These mutants can also be an important tool for drug discovery as they can be a great option for high-throughput drug screens. In the next section, we will learn about some genetic research studies that have used C. elegans as a model organism.
The complete genome sequence of C. elegans was published in 1998. This nematode worm was the first multicellular organism to have its whole genome sequenced. Its genome is about 100 million base pairs in length and contains about 20,000 protein coding genes, which is a similar number of genes as humans.
Many genes known to be associated with human disease have a counterpart in C. elegans, making it a very useful model for disease research. C. elegans mutants have provided models for many neurological, cardiac and kidney diseases in humans. These mutants can also be an important tool for drug discovery as they can be a great option for high-throughput drug screens. In the next section, we will learn about some genetic research studies that have used C. elegans as a model organism.
C. elegans as a Model Organism for Gene Function Research
The publication of Brenner’s landmark paper on C. elegans genetics marked its beginning as a model organism for research. Since then, several significant discoveries have resulted in Nobel prizes for C. elegans researchers. Sydney Brenner, Robert Horvitz, and John Sulston received the prize for their discoveries related to cell death machinery; Andrew Fire and Craig Mello for their discovery of RNAi; and Osamu Shimomura, Martin Chalfie, and Roger Tsien for the discovery of the green fluorescence protein.
Recently, RNA interference (RNAi) technique has become an essential tool in C. elegans research by revolutionizing the process of making worm mutants. RNAi is a rapid and simple method for determining loss-of-function phenotypes of genes and has been used in genome-wide screens in C. elegans.
The use of RNAi technology for C. elegans as a model has allowed researchers to study several biological mechanisms. Genetic modifications of this nematode worm have contributed to research in aging and the understanding of neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease. RNAi screening in the nematode has also led to the identification of genes that control fat accumulation, thus providing scientists with a deeper understanding of obesity in humans.
Although RNAi has been a powerful tool for genetic research, it only reduces gene expression at the mRNA level instead of permanently silencing the gene. To overcome this issue, scientists have started using the novel gene-editing tool––CRISPR for genetic research. Due to its many advantages, CRISPR technology has the potential to surpass RNAi in biological research. This novel technology is quickly becoming an indispensable gene-editing tool in many model organisms including C. elegans.
Genome Modifications in C. Elegans using CRISPR
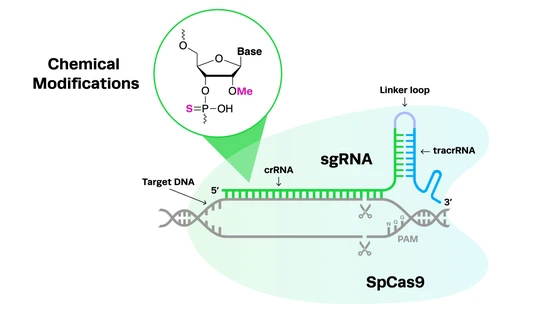
CRISPR-Cas9 is a novel genome editing tool that has led to a revolution in genetic research since its discovery. It has enabled scientists to edit and manipulate DNA in a rapid, accurate and cost-effective way. Some of the CRISPR-based methods used for making genome modifications in C. elegans are described below.
Using CRISPR-Cas9 to elicit chromosomal rearrangements in C. elegansThere are several genes that play an important role in fertility and development. The disruption of these genes leads to arrested growth or sterility. Genetic balancers are chromosomal rearrangements that allow sterile mutations to be stably maintained and have been widely used to study essential genes in organisms. However, generating and maintaining these mutations can be a challenging process.
A team of researchers has found an easy and efficient way to generate and maintain loss-of-function alleles of essential genes using CRISPR-Cas9 technology. They developed a method to induce chromosomal translocations and produce genetic balancers using CRISPR and used this approach to edit essential genes in C. elegans.
There are several genes that play an important role in fertility and development. The disruption of these genes leads to arrested growth or sterility. Genetic balancers are chromosomal rearrangements that allow sterile mutations to be stably maintained and have been widely used to study essential genes in organisms. However, generating and maintaining these mutations can be a challenging process.
A team of researchers has found an easy and efficient way to generate and maintain loss-of-function alleles of essential genes using CRISPR-Cas9 technology. They developed a method to induce chromosomal translocations and produce genetic balancers using CRISPR and used this approach to edit essential genes in C. elegans.
Using CRISPR-Cas9 to generate putative null mutants in Caenorhabditis elegans Null mutants are necessary to understand gene function. Many of the C. elegans mutants generated in the past have genes that have only reduction-of-function alleles or no mutant alleles at all. Hence, it is of great importance to find and easy and efficient method to generate null mutants in the wild-type.
A team of scientists has described a simple and effective method to generate null mutants in C. elegans using CRISPR-Cas9 and short single-stranded DNA oligo repair templates to insert a STOP-IN cassette into the early exons of target genes. The team hopes that this method will facilitate the generation of new null mutants to analyze gene function in C. elegans and other model organisms.
Apart from the ones discussed above, there are several other CRISPR-based approaches that have been identified to make genetic modifications in C. elegans. To learn more about these methods, you can refer to this review article published in GENETICS.
We hope you found this post useful. If you are interested in learning more about CRISPR, be sure to follow CRISPR in the news. You can also keep up with the latest CRISPR news by subscribing to our blog, or by following us on Twitter or Facebook!
Null mutants are necessary to understand gene function. Many of the C. elegans mutants generated in the past have genes that have only reduction-of-function alleles or no mutant alleles at all. Hence, it is of great importance to find and easy and efficient method to generate null mutants in the wild-type.
A team of scientists has described a simple and effective method to generate null mutants in C. elegans using CRISPR-Cas9 and short single-stranded DNA oligo repair templates to insert a STOP-IN cassette into the early exons of target genes. The team hopes that this method will facilitate the generation of new null mutants to analyze gene function in C. elegans and other model organisms.
Apart from the ones discussed above, there are several other CRISPR-based approaches that have been identified to make genetic modifications in C. elegans. To learn more about these methods, you can refer to this review article published in GENETICS.
We hope you found this post useful. If you are interested in learning more about CRISPR, be sure to follow CRISPR in the news. You can also keep up with the latest CRISPR news by subscribing to our blog, or by following us on Twitter or Facebook!