Contents
Cancer is the second most common cause of death globally, and despite years of research, the progress towards cures may seem slow. Until the advent of CRISPR, a tool that has certainly accelerated cancer research. In this blog post, we’ll explore why a cure for cancer seems elusive, discuss a variety of cellular immunotherapies for cancer, and how CRISPR has improved their safety and efficacy. We will also cover CRISPR-editing in vivo for solid tumors, the current landscape of CRISPR cancer research and trials, and the future of CRISPR cancer therapeutics.
Why is Cancer So Difficult to Treat?
Scientists have made huge progress in cancer research in recent years, but a cure remains elusive. This is because cancer is an incredibly complex group of diseases; there are hundreds of different types of cancer, each with a unique signature of genetic mutations. These mutations either switch on oncogenes or switch off tumor-suppression genes, either of which leads to unchecked (cancerous) growth of cells.
The genetic profile is distinct not only between cancer types—for example, between lung and liver cancers—but also within each type, and even within tumors themselves. Among breast cancer patients, for example, it’s unlikely that any two tumors will possess the exact same profile of genetic mutations. The complex tumor microenvironment (TME) is immunosuppressive and is comprised of many different types of cells which also have different mutation profiles.
This genetic complexity influences how aggressive cancers are, how they will respond to various treatments, and whether they will re-occur. This means that there can never be a ‘one-size-fits-all’ cure, and generating accurate models for research is challenging. Additionally, many types of cancer are difficult to treat due to current screening and diagnostic limitations. In some cases, cancer is not diagnosed until it has metastasized and spread to multiple organs.
Once cancer has metastasized, it is more difficult to treat, partly because metastatic tumors are likely to have additional mutations compared to the original tumor and will therefore respond to treatment differently. The accruement of mutations over time can also cause cancers to become drug-resistant. Cancer cells are also very adept at avoiding and/or suppressing the immune system in order to continue to grow. Some cancers are malignancies of the immune cells themselves. For example, lymphoma is a type of cancer that affects lymphocytes, the immune cells that would normally fight cancer and infections.
CRISPR: A Game-Changer in Cancer Therapeutics
Adoptive cell therapies (ACTs) for the treatment of cancer, like TIL, TCR, CAR-T and NK, are desirable over traditional treatments such as chemotherapy and radiotherapy because they are more targeted to cancerous tissue, leaving healthy cells untouched. All cell-based immunotherapies involve expanding (growing) these immune cells ex vivo and re-infusing them into patients so their body has more immune cells available to attack the cancer. These ACTs can be autologous (isolated from the patient) or allogeneic (isolated from a healthy donor).
While all ACTs have encountered various hurdles in their translation into the clinic, in recent years CRISPR has offered new hope for ACT-based cancer treatment. In this section, we’ll explore the main types of cellular immunotherapies used to treat cancer, how CRISPR can be used to make them stronger, safer, and more readily available, and how CRISPR can target cancer directly.
TIL and TCR cell therapiesT cells, often called T lymphocytes, encompass several subsets of cells and are a key component of the adaptive immune system. As their name suggests, tumor-infiltrating lymphocytes (TILs) are immune cells that can invade tumors in order to kill cancer cells. Autologous TIL therapy involves extracting TILs from a patient’s tumor, expanding them in large numbers ex vivo, and re-infusing them into the patient so they have more TILs available to fight cancer. Despite the early promise of TIL therapy, issues with the isolation and ex vivo expansion of TILs, along with their relatively limited ability to destroy tumors, eventually led to this approach being sidelined in favor of T-cell receptor (TCR) therapy.
Think of TCR therapy as an upgrade to TIL therapy. TCR therapy uses T cells that are genetically modified to express specific TCRs on their surface, making them better at recognizing cancer cells.
TCR therapy showed promise, but like TILs, it encountered a few obstacles in its clinical translation. The lack of specificity of TCRs led to problems with the clinical translation of TCR therapy because cancer cells can downregulate the expression of MHC to evade detection by TCR cells. Expression of endogenous TCRs alongside the transgenic TCR in the cells is also a key problem, causing competition for signaling components, the formation of heterodimers that can cause lethal autoimmunity, and graft-versus-host disease (GvHD). GvHD, a condition where grafts of healthy immune cells recognize the patient’s own cells and tissues as foreign and attack them, is a major concern in any form of allogeneic (non-self) cellular immunotherapy and can be lethal.
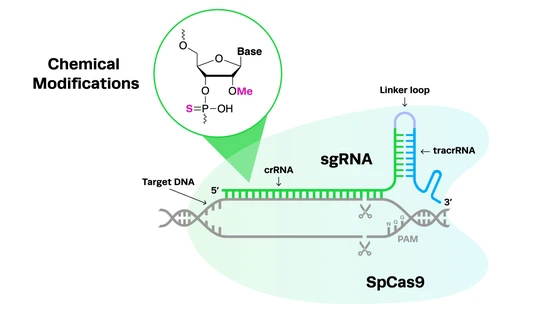
Before the advent of CRISPR, engineering T cells was time-consuming, difficult, and expensive. Safety concerns associated with the use of retroviral and lentiviral vectors for editing were also a limiting factor. Fortunately, the recent CRISPR revolution has led to a revival of both TIL and TCR cell therapies. In TILs, CRISPR can be used to knock out a gene that inhibits T cell function called cytokine-induced SH2 (CISH) - disruption of CISH increases TIL aggression towards tumors.
In the case of TCR cells, CRISPR can be used for knock-in of the required TCR to specific sites in the T cell genome to increase its expression. It can also be used to knock out the endogenous TCR gene that can cause GvHD and other adverse events. Moreover, CRISPR can generate these edits in very short timeframes and without the use of viral vectors, increasing patient safety outcomes.
T cells, often called T lymphocytes, encompass several subsets of cells and are a key component of the adaptive immune system. As their name suggests, tumor-infiltrating lymphocytes (TILs) are immune cells that can invade tumors in order to kill cancer cells. Autologous TIL therapy involves extracting TILs from a patient’s tumor, expanding them in large numbers ex vivo, and re-infusing them into the patient so they have more TILs available to fight cancer. Despite the early promise of TIL therapy, issues with the isolation and ex vivo expansion of TILs, along with their relatively limited ability to destroy tumors, eventually led to this approach being sidelined in favor of T-cell receptor (TCR) therapy.
Think of TCR therapy as an upgrade to TIL therapy. TCR therapy uses T cells that are genetically modified to express specific TCRs on their surface, making them better at recognizing cancer cells.
TCR therapy showed promise, but like TILs, it encountered a few obstacles in its clinical translation. The lack of specificity of TCRs led to problems with the clinical translation of TCR therapy because cancer cells can downregulate the expression of MHC to evade detection by TCR cells. Expression of endogenous TCRs alongside the transgenic TCR in the cells is also a key problem, causing competition for signaling components, the formation of heterodimers that can cause lethal autoimmunity, and graft-versus-host disease (GvHD). GvHD, a condition where grafts of healthy immune cells recognize the patient’s own cells and tissues as foreign and attack them, is a major concern in any form of allogeneic (non-self) cellular immunotherapy and can be lethal.
Before the advent of CRISPR, engineering T cells was time-consuming, difficult, and expensive. Safety concerns associated with the use of retroviral and lentiviral vectors for editing were also a limiting factor. Fortunately, the recent CRISPR revolution has led to a revival of both TIL and TCR cell therapies. In TILs, CRISPR can be used to knock out a gene that inhibits T cell function called cytokine-induced SH2 (CISH) - disruption of CISH increases TIL aggression towards tumors.
In the case of TCR cells, CRISPR can be used for knock-in of the required TCR to specific sites in the T cell genome to increase its expression. It can also be used to knock out the endogenous TCR gene that can cause GvHD and other adverse events. Moreover, CRISPR can generate these edits in very short timeframes and without the use of viral vectors, increasing patient safety outcomes.
CAR-T cell therapy
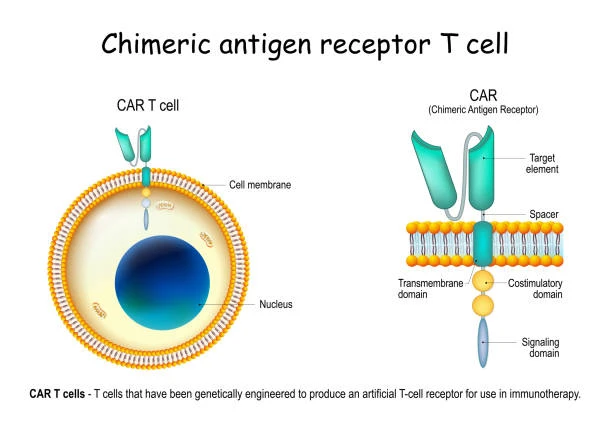
CAR-T therapy is a gene-edited cell therapy, whereby T cells are edited to express a specific chimeric antigen receptor (CAR) on their surface. CRISPR-based CAR-T therapies can be developed The CAR is specific to the type of cancer the patient has, allowing the T cells to recognize the antigens produced by the cancer cells and kill them. Importantly, CAR does not rely on the presence of MHC in order to recognize and kill cancer cells.
CAR-T therapies, both autologous (patient-derived) and allogeneic (healthy donor-derived), currently have some major limitations. For autologous CAR-T, this includes difficulty procuring large numbers of T cells from patients that are lymphopenic due to other treatments and long timeframes for generating a sufficient therapeutic dose. For allogeneic CAR-T, the healthy donor-derived cells can be rejected by the patient immune system, cause toxicity, or induce GvHD.
CAR T Therapy: Treating Cancer with Engineered Cells (with Podcast)
Synthego sits down with Avery Posey, leading the charge on CAR T cell therapy research and bringing this technology to cancer patients as soon as possible. Learn how CAR T works, what stage it’s in now, and how it will help revolutionize the treatment of cancer.

Unfortunately, some cancers are able to avoid destruction by CAR-T cells by overexpressing certain proteins, like programmed cell death protein 1 (PD-1), on their surface.
Developing CAR-T cells using CRISPR involves cutting-edge nucleases and CAR-T guide RNA. Using CRISPR has resulted in increased safety and efficacy of CAR-T cell therapies in a variety of ways. Firstly, CRISPR can be used to knock in the CAR in a targeted manner - for example, to a safe harbor site in the genome - to ensure sufficient, long-term expression of the receptor on the cell surface. Secondly, CRISPR can knock out certain genes in CAR-T cells to increase their cancer-killing activity. Thirdly, it can be used to create edits that minimize CAR-T cell exhaustion and increase their long-term proliferation. Finally, CRISPR edits can be used to generate universal, ‘off-the-shelf’ CAR-T cells from induced pluripotent stem cells (iPSCs), negating harvesting limitations, long wait times, and toxicity issues.
For an in-depth discussion of this strategy, you can read our blog on how CRISPR has revolutionized CAR-T therapy for cancer treatment.
CRISPR CAR-T cells: Edited T Cells Are Revolutionizing Cancer Treatment
CAR-T cell therapy is a form of immunotherapy used to treat cancer. CRISPR-Cas9 gene editing has recently been used to increase the safety and efficacy of CAR-T therapies. This article explores CAR-T therapy, how CRISPR can be used to improve CAR-T, and how CRISPR-edited CAR-T cells are revolutionizing cancer treatment.
CAR-T vs. TCR therapy: What’s the difference?The main difference between CAR-T and TCR therapy is the receptors used and their mode of action. Although they sound similar, CAR-T and TCR work very differently. TCRs are naturally-occurring or minimally modified receptors, which rely on major histocompatibility complex (MHC) proteins to work and are therefore non-specific. In contrast, CARs are artificial receptors that are engineered to recognize specific cancer antigens.
Unlike TCRs, which can recognize extracellular and intracellular components, CARs can only recognize extracellular molecules on cancer cells, meaning the range of targets of CAR-T therapy is less than TCR. CAR-T cells also require more antigens to be present in order to be activated compared to TCR cells. Like other cell therapies, CAR-T cells are prone to becoming ‘exhausted’, a state in which their efficacy is reduced.
The main difference between CAR-T and TCR therapy is the receptors used and their mode of action. Although they sound similar, CAR-T and TCR work very differently. TCRs are naturally-occurring or minimally modified receptors, which rely on major histocompatibility complex (MHC) proteins to work and are therefore non-specific. In contrast, CARs are artificial receptors that are engineered to recognize specific cancer antigens.
Unlike TCRs, which can recognize extracellular and intracellular components, CARs can only recognize extracellular molecules on cancer cells, meaning the range of targets of CAR-T therapy is less than TCR. CAR-T cells also require more antigens to be present in order to be activated compared to TCR cells. Like other cell therapies, CAR-T cells are prone to becoming ‘exhausted’, a state in which their efficacy is reduced.
NK cell therapyNatural killer (NK) cells are major players in the immune system, just like T cells. One of the key differences between these cell types is that NK cells are part of the innate immune system, while T cells are a component of the adaptive immune system. NK cells perform immune surveillance, patrolling the bloodstream for abnormal cells such as cancer cells, bacteria, or virus-infected cells. Unlike T cells, NK cells are not antigen-specific and they simply recognize ‘non-self’ cells, regulated by both activating and inhibitory receptors expressed on their surface. When activated, NK cells release cytotoxic molecules that lyse the non-self cell and kill it.
Natural killer (NK) cells are major players in the immune system, just like T cells. One of the key differences between these cell types is that NK cells are part of the innate immune system, while T cells are a component of the adaptive immune system. NK cells perform immune surveillance, patrolling the bloodstream for abnormal cells such as cancer cells, bacteria, or virus-infected cells. Unlike T cells, NK cells are not antigen-specific and they simply recognize ‘non-self’ cells, regulated by both activating and inhibitory receptors expressed on their surface. When activated, NK cells release cytotoxic molecules that lyse the non-self cell and kill it.
NK cell therapy is excellent at killing cancer cells and does not cause GvHD, which is a common side effect of other allogeneic immune cell transplants like CAR-T. However, there are several key problems that have prevented their widespread use. This includes issues with the ex vivo expansion of NK cells for clinical use, their relatively short lifespan and low persistence in vivo, and a limited ability to infiltrate solid tumors. Some types of cancer also manage to develop mutations that help them evade NK cells.
Similar to the recent progress in CAR-T cell therapies, CRISPR can be used to enhance NK cell therapies. For example, like T cells, NKs can also be edited to express a CAR (CAR-NK). Along with their innate non-self recognition, the addition of a CAR means they can work both specifically and non-specifically, reducing the likelihood that cancer cells will evade treatment. CRISPR can also be used to create edits that increase the lifespan of these typically short-lived cells or to enhance their cancer-targeting ability.
CRISPR gene editing in vivo in tumorsIn addition to gene-edited cell therapies, like CAR-T, NK, TCR, and TIL, CRISPR-Cas9 can also be used to target tumors directly in vivo. Genetic mutations that switch on oncogenes (gain-of-function mutations) stimulate carcinogenesis, and the expression of these oncogenes is specific to cancer cells. Knocking out these genes via CRISPR-Cas9 is an appealing therapeutic target because it will prevent cancer growth. Recent examples include a lipid nanoparticle (LNP)-based delivery approach to disrupt the overexpressed PLK1 gene, and a lentivirus delivery strategy to target multiple cancer-specific indels.
In some cases, this can be a bit tricky - for example, when attempting to disrupt fusion oncogenes (FOs), which are chimeric oncogenes resulting from chromosomal rearrangements. However, recent studies have developed strategies to target two introns of the genes involved in these rearrangements using CRISPR-Cas9. In cancers caused by FOs, this targeted disruption causes apoptosis of the malignant cells and eliminates the possibility of further growth.
For more information on how CRISPR is being used in cancer research and treatment, you can listen to our recent CRISPR Cuts podcast with Dr. Jesse Boehm, who speaks about his research on rare cancers and his new foundation, Break Through Cancer.
In addition to gene-edited cell therapies, like CAR-T, NK, TCR, and TIL, CRISPR-Cas9 can also be used to target tumors directly in vivo. Genetic mutations that switch on oncogenes (gain-of-function mutations) stimulate carcinogenesis, and the expression of these oncogenes is specific to cancer cells. Knocking out these genes via CRISPR-Cas9 is an appealing therapeutic target because it will prevent cancer growth. Recent examples include a lipid nanoparticle (LNP)-based delivery approach to disrupt the overexpressed PLK1 gene, and a lentivirus delivery strategy to target multiple cancer-specific indels.
In some cases, this can be a bit tricky - for example, when attempting to disrupt fusion oncogenes (FOs), which are chimeric oncogenes resulting from chromosomal rearrangements. However, recent studies have developed strategies to target two introns of the genes involved in these rearrangements using CRISPR-Cas9. In cancers caused by FOs, this targeted disruption causes apoptosis of the malignant cells and eliminates the possibility of further growth.
For more information on how CRISPR is being used in cancer research and treatment, you can listen to our recent CRISPR Cuts podcast with Dr. Jesse Boehm, who speaks about his research on rare cancers and his new foundation, Break Through Cancer.
The Current Landscape of CRISPR Cancer Therapeutics

The field of CRISPR cancer therapy is moving at a rapid pace, with lots of exciting proof-of-concept and pre-clinical studies being published regularly, the results of the first clinical trials starting to trickle in, and many new trials beginning. In this section, we’ll take a look at the current state of CRISPR cancer research and therapy, including the types of cancer that can be treated, pre-clinical studies and clinical trials, and therapies that have been approved by the FDA.
Hematological cancers versus solid tumorsHematological (blood) cancers, like leukemia, lymphoma, and multiple myeloma, present very different treatment challenges compared to solid tumors in organs or soft tissue, like breast, lung, or brain cancers. In general, gene-edited cell therapies for hematological malignancies are currently more advanced than those for solid tumors.
While tumors can often be treated surgically, blood cancers cannot be removed surgically because they involve malignant cells circulating freely in the body. However, many tumors cannot be surgically removed, or can only be partially removed. In these cases, treatment options become more difficult because of the complex TME and its immunosuppressive effects.
CAR-T therapies were originally developed to treat blood cancers because it is easier for T cells to attack freely circulating cancer cells rather than tumors, while TILs can be used to target solid tumors. In all cases, CRISPR is being used to enhance these therapies, increasing their safety and efficacy, and reducing their costs and production times.
Hematological (blood) cancers, like leukemia, lymphoma, and multiple myeloma, present very different treatment challenges compared to solid tumors in organs or soft tissue, like breast, lung, or brain cancers. In general, gene-edited cell therapies for hematological malignancies are currently more advanced than those for solid tumors.
While tumors can often be treated surgically, blood cancers cannot be removed surgically because they involve malignant cells circulating freely in the body. However, many tumors cannot be surgically removed, or can only be partially removed. In these cases, treatment options become more difficult because of the complex TME and its immunosuppressive effects.
CAR-T therapies were originally developed to treat blood cancers because it is easier for T cells to attack freely circulating cancer cells rather than tumors, while TILs can be used to target solid tumors. In all cases, CRISPR is being used to enhance these therapies, increasing their safety and efficacy, and reducing their costs and production times.
Current preclinical research and clinical trialsCurrent CRISPR cancer research includes engineering TILs to be resistant to the molecules that cancer cells express in order to switch lymphocytes off, the creation of universal, off-the-shelf CAR-T cells, and the editing of NK cells for better cancer targeting. For an in-depth look at the different ways CRISPR can be used for cancer immunotherapy, check out this 2021 review.
In a clinical setting, current CRISPR-based trials include treatments for non-small-cell lung cancer (NSCLC), esophageal cancer, cervical cancer, metastatic gastrointestinal cancer, T- and B-cell malignancies, multiple myeloma, melanoma, and acute myeloid leukemia (AML), to name a few. For a full breakdown of the current state of clinical trials for T cell therapies, you can read this 2021 review from the Journal of Experimental & Clinical Cancer Research.
CRISPR Therapeutics is one of the companies leading the charge on gene-edited T cell therapies and is currently developing off-the-shelf, allogeneic CAR-T cells using CRISPR technology. In December 2021, CRISPR Therapeutics also announced a new partnership with NK-specialists Nkarta Therapeutics in order to develop CRISPR-edited NK cell therapy.
Intellia Therapeutics, who were already working on CAR-T therapies, announced a collaboration with ONK Therapeutics to work on several different CRISPR-edited NK therapies of cancer. The FDA has also recently fast-tracked a CRISPR-edited TCR cell therapy for the treatment of AML from Intellia Therapeutics, NTLA-5001.
In a pre-clinical study, Editas Medicine has developed iPSC-derived NK cells (iNK), using CRISPR-Cas12a to knock-in genes that increase NK cell persistence and tumor-killing capacity. Some excellent resources for staying up-to-date with current research and clinical trials using CRISPR technology include the Innovative Genomics Institute website and the CRISPR Medicine News clinical trials page.
For a valuable patient perspective on understanding cancer and getting treatment, you can listen to our interview with Bryce Olson on CRISPR Cuts podcast. Bryce’s journey, from being diagnosed with aggressive prostate cancer, to learning about the science of cancer treatment and pursuing genome sequencing and personalized cell therapies, is both interesting and inspiring.
Current CRISPR cancer research includes engineering TILs to be resistant to the molecules that cancer cells express in order to switch lymphocytes off, the creation of universal, off-the-shelf CAR-T cells, and the editing of NK cells for better cancer targeting. For an in-depth look at the different ways CRISPR can be used for cancer immunotherapy, check out this 2021 review.
In a clinical setting, current CRISPR-based trials include treatments for non-small-cell lung cancer (NSCLC), esophageal cancer, cervical cancer, metastatic gastrointestinal cancer, T- and B-cell malignancies, multiple myeloma, melanoma, and acute myeloid leukemia (AML), to name a few. For a full breakdown of the current state of clinical trials for T cell therapies, you can read this 2021 review from the Journal of Experimental & Clinical Cancer Research.
CRISPR Therapeutics is one of the companies leading the charge on gene-edited T cell therapies and is currently developing off-the-shelf, allogeneic CAR-T cells using CRISPR technology. In December 2021, CRISPR Therapeutics also announced a new partnership with NK-specialists Nkarta Therapeutics in order to develop CRISPR-edited NK cell therapy.
Intellia Therapeutics, who were already working on CAR-T therapies, announced a collaboration with ONK Therapeutics to work on several different CRISPR-edited NK therapies of cancer. The FDA has also recently fast-tracked a CRISPR-edited TCR cell therapy for the treatment of AML from Intellia Therapeutics, NTLA-5001.
In a pre-clinical study, Editas Medicine has developed iPSC-derived NK cells (iNK), using CRISPR-Cas12a to knock-in genes that increase NK cell persistence and tumor-killing capacity. Some excellent resources for staying up-to-date with current research and clinical trials using CRISPR technology include the Innovative Genomics Institute website and the CRISPR Medicine News clinical trials page.
For a valuable patient perspective on understanding cancer and getting treatment, you can listen to our interview with Bryce Olson on CRISPR Cuts podcast. Bryce’s journey, from being diagnosed with aggressive prostate cancer, to learning about the science of cancer treatment and pursuing genome sequencing and personalized cell therapies, is both interesting and inspiring.
The Future of CRISPR Cancer Therapeutics
Scientists are hopeful that the use of both gene-edited cell therapies and in vivo CRISPR gene editing will enable the treatment of many types of cancer. However, we’re only just beginning to scratch the surface of what CRISPR is able to do in this area of research. Recent adaptations of CRISPR technology, including newly discovered Cas nucleases, are revealing even more avenues for cancer therapeutics.
For example, the discovery of compact type I-C Cascade-Cas3 systems offers the possibility of creating large-scale deletions in tumor survival genes in order to kill cancer, because Cas3 shreds large sections of DNA rather than creating double-stranded breaks. Other adaptations of CRISPR systems that may prove therapeutically relevant to cancer treatment include CRISPR-based epigenome editing (eGE), which can be used to switch on tumor suppressor genes or switch off oncogenes, or otherwise alter epigenetic alterations associated with cancer.
With a combination of current and emerging CRISPR technologies for the treatment of cancer, we’re likely to see a rapid increase in pre-clinical studies and clinical trials over the next few years. As the results of the ongoing trials continue to show promise, and CRISPR systems are further refined in terms of safety and efficacy, the development of cancer cures finally seems within reach.