Contents
CRISPR Cuts is Synthego’s official podcast, where scientists-turned-marketers Kevin Bryant and Minu Prabhune discuss what’s going on in CRISPR news with the leading experts in the field. In this episode, we sat down with Trevor Martin of Mammoth Biosciences to get the inside scoop on one of the latest developments in CRISPR.
Mammoth Biosciences, one of the top CRISPR startup companies (co-founded by Jennifer Doudna herself!), works on an amazing application of CRISPR technology in diagnostics. In this podcast, Martin explains how CRISPR can be used in diagnostics, and what Mammoth is doing to bring it straight to the hands of consumers.
What is CRISPR Diagnostics Technology and How Does It Work?
CRISPR diagnostics involves application of the genome engineering tool for detecting diseases, both infectious and non-infectious. Companies like Mammoth Biosciences are aiming to develop diagnostic tests that can detect major diseases using CRISPR from pieces of genetic material circulating in blood, urine, or saliva.
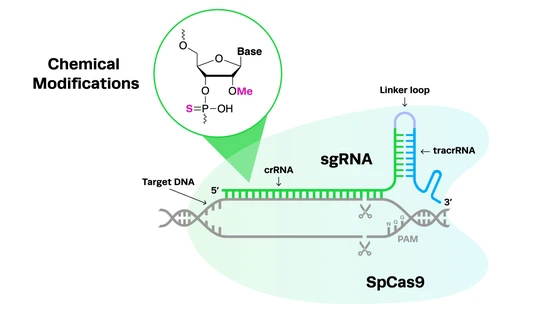
Similar to Cas9, Cas12 cuts DNA, while Cas13 targets RNA. But Cas12 and Cas13 possess a special ability (unlike Cas9). Once the guide RNA finds the target sequence, these nucleases cut any single stranded DNA strands in the vicinity.
In CRISPR diagnostic kits, fluorescent reporters are attached to ssDNA strands, so that when the Cas enzyme cuts the ssDNA, it activates the reporter, causing it to fluoresce or change color, thus enabling visual detection of the specific disease nucleic marker.
In theory, these diagnostic tools could be as small as at-home pregnancy tests, and could even work with smartphone apps, because of the recent ability to move these tests to strips. This is a significant improvement on medical diagnostics for typical consumers; this technology would bring results much faster, for far less money, and would require fewer trained professionals to administer them.
In more detail: CRISPR is popular for its “cut and paste” function. When a guide RNA finds a target DNA sequence, the Cas nuclease makes a double-stranded cut. While Cas9 is the most popular CRISPR nuclease, newer nucleases such as Cas12 and Cas13 have enabled researchers to use the “search” function of CRISPR in diagnostics.
How Mammoth Biosciences Got its Start
In this section, Martin provides insight on how Mammoth Biosciences was founded, and the ideals behind what they were trying to build at the company.
Minu: So could you start by telling us about your background and how you ended up co-founding Mammoth?Trevor Martin: I'm originally from Georgia and was there for the first 18 years of my life, and I first got interested in science actually in college at Princeton. They had a really cool program there called Integrated Science where they combined all the disciplines together like math, physics, chemistry, biology. But what I really focused on was biology and I really kind of fell in love with that idea of a kind of cross-disciplinary work and that really set things up for when I went over to grad school at Stanford and started working in computational biology.
But towards the end of my graduate school, I got very interested in emerging technologies for diagnostics and saw diagnostics as a field where there's a lot of opportunities for core innovation. A lot of what’s used in the field have been very similar for a very long time. There's a lot of great technologies, of course, like PCR, but I think it was just right for a new way of thinking about it fundamentally.
So, initially, not even on a specific technology, but just different ways of thinking about diagnostics that could really enable new modes and new ways of accessing that kind of molecular information. Whether that's at home or in the hospital or in the doctor's office.
So, originally, actually Mammoth as it is today was founded as another company called Apheleia by myself and another Ph.D. from Stanford, Ashley Tehranchi, just with this kind of general thesis that there's got to be a better more successful and more democratized way of accessing molecular information in the form of diagnostics. So we initially founded it with that thesis and around the same time, and interestingly enough, Jennifer Doudna's lab very quickly had many huge advances in developing these CRISPR tools that's something that could be used as a diagnostic.
So when we saw these papers, we were immediately really excited because it really fit that thesis that we had and it was really great timing since they were developing these tools. So we reached out to Jennifer and her lab. The two, at the time, graduate students who were there working on these systems, Lucas Harrington and Janice Chen, were super entrepreneurial and when we met them we were just blown away by how much their vision aligned with ours and how excited they also were about leveraging these types of systems for creating really awesome diagnostic tests.
So we teamed up with them and with Jennifer and that's when we really formed what you now know as Mammoth BioSciences. The co-founding team now is five people and that's when we really hit the ground running in terms of developing new systems that we're excited about today.
Trevor Martin: I'm originally from Georgia and was there for the first 18 years of my life, and I first got interested in science actually in college at Princeton. They had a really cool program there called Integrated Science where they combined all the disciplines together like math, physics, chemistry, biology. But what I really focused on was biology and I really kind of fell in love with that idea of a kind of cross-disciplinary work and that really set things up for when I went over to grad school at Stanford and started working in computational biology.
But towards the end of my graduate school, I got very interested in emerging technologies for diagnostics and saw diagnostics as a field where there's a lot of opportunities for core innovation. A lot of what’s used in the field have been very similar for a very long time. There's a lot of great technologies, of course, like PCR, but I think it was just right for a new way of thinking about it fundamentally.
So, initially, not even on a specific technology, but just different ways of thinking about diagnostics that could really enable new modes and new ways of accessing that kind of molecular information. Whether that's at home or in the hospital or in the doctor's office.
So, originally, actually Mammoth as it is today was founded as another company called Apheleia by myself and another Ph.D. from Stanford, Ashley Tehranchi, just with this kind of general thesis that there's got to be a better more successful and more democratized way of accessing molecular information in the form of diagnostics. So we initially founded it with that thesis and around the same time, and interestingly enough, Jennifer Doudna's lab very quickly had many huge advances in developing these CRISPR tools that's something that could be used as a diagnostic.
So when we saw these papers, we were immediately really excited because it really fit that thesis that we had and it was really great timing since they were developing these tools. So we reached out to Jennifer and her lab. The two, at the time, graduate students who were there working on these systems, Lucas Harrington and Janice Chen, were super entrepreneurial and when we met them we were just blown away by how much their vision aligned with ours and how excited they also were about leveraging these types of systems for creating really awesome diagnostic tests.
So we teamed up with them and with Jennifer and that's when we really formed what you now know as Mammoth BioSciences. The co-founding team now is five people and that's when we really hit the ground running in terms of developing new systems that we're excited about today.
Kevin: So when did the original company get started and when did you guys kind of change direction into the company you are now?Trevor: Well it's not ancient history - it was just last year, in 2017. So the company's young no matter how you place it, for sure. It’s exciting and moving very quickly and we're excited to be at the forefront of that, that's why I think it's always exciting in our field.
Trevor: Well it's not ancient history - it was just last year, in 2017. So the company's young no matter how you place it, for sure. It’s exciting and moving very quickly and we're excited to be at the forefront of that, that's why I think it's always exciting in our field.
Realizing the Potential of CRISPR For Diagnostic Applications
Kevin: I think CRISPR is most probably well known for its ability to very specifically, precisely, and relatively easily edit certain DNA sequences, but that's not all it can do. So your company is fairly unique in that you focus on one, in other areas that this technology can be used, other applications for it but sort of non-editing applications of CRISPR. So can you tell us a little bit more about the work you are doing at your company and the ways you're using CRISPR to take the technology into new areas?Trevor: Yeah, I mean that's a great point. And I think one of the things that makes us really excited about what we're doing at Mammoth is that we see it as a fundamental shift in thinking about what CRISPR is.
Because as you mentioned, usually when people think about CRISPR you think about it as a gene editing tool and it is an amazing gene editing tool, that's not a bad thing to think about it as because it is a shocking tool. But really when we took a step back and we started evaluating what CRISPR is and what we need, we started thinking about CRISPR being control F, rather than control X.
There are a lot of ways to cut nucleic acids, but one of the real powers of CRISPR is being able to find specific sequences. And of course you can cut them or do other things, but we really think of CRISPR fundamentally as a kind of search engine for biology; like Google for biology, rather than kind of specifically a word processing tool - although it's really good at that too. We just take this different perspective on it as something that can be used to search and find things and you can do lots of cool stuff with it once you find but then really the power there is being able to locate very specific items.
That's kind of how we think about it. So, when you take that fundamental way of viewing CRISPR and you start thinking about application areas then, at least for us, it becomes really obvious the diagnostics is a very exciting area, right? Because that's, fundamentally, a search problem and that's is something that CRISPR is really great at so we've been leveraging these proteins to build diagnostic tools that can be programmed the same way that you program a CRISPR protein to edit something. But now, instead of editing it, you can program it to find something, and then to tell you that result. Whether that's an infectious disease, we have some sort of sequence that's specific to malaria, tuberculosis, or maybe even like a specific step to figure out what the genotype is and look at some of the genomes. That's kind of the way that we view CRISPR at Mammoth.
Trevor: Yeah, I mean that's a great point. And I think one of the things that makes us really excited about what we're doing at Mammoth is that we see it as a fundamental shift in thinking about what CRISPR is.
Because as you mentioned, usually when people think about CRISPR you think about it as a gene editing tool and it is an amazing gene editing tool, that's not a bad thing to think about it as because it is a shocking tool. But really when we took a step back and we started evaluating what CRISPR is and what we need, we started thinking about CRISPR being control F, rather than control X.
There are a lot of ways to cut nucleic acids, but one of the real powers of CRISPR is being able to find specific sequences. And of course you can cut them or do other things, but we really think of CRISPR fundamentally as a kind of search engine for biology; like Google for biology, rather than kind of specifically a word processing tool - although it's really good at that too. We just take this different perspective on it as something that can be used to search and find things and you can do lots of cool stuff with it once you find but then really the power there is being able to locate very specific items.
That's kind of how we think about it. So, when you take that fundamental way of viewing CRISPR and you start thinking about application areas then, at least for us, it becomes really obvious the diagnostics is a very exciting area, right? Because that's, fundamentally, a search problem and that's is something that CRISPR is really great at so we've been leveraging these proteins to build diagnostic tools that can be programmed the same way that you program a CRISPR protein to edit something. But now, instead of editing it, you can program it to find something, and then to tell you that result. Whether that's an infectious disease, we have some sort of sequence that's specific to malaria, tuberculosis, or maybe even like a specific step to figure out what the genotype is and look at some of the genomes. That's kind of the way that we view CRISPR at Mammoth.
Cas12 and Cas13 Nucleases at the Core of the Workflow
How are these new nucleases in the Cas family leading the way in CRISPR diagnostic applications in the field of medicine?
Minu: I think that many people who are not completely familiar with CRISPR probably still think of CRISPR as, you know, CRISPR-Cas9 because Cas9 has played such a main role in the CRISPR system that we tend to forget that there are so many other new places out there. One thing I found really interesting is that your whole diagnostic platform is based on two different nucleuses other than Cas9, right? So, could you just tell us briefly how the diagnosis would work if you have a sample and then what your kit would do?Trevor: Yeah, so the two proteins right now are Cas12 and Cas13, so Cas12 for searching through DNA and Cas13 for RNA. And the way it works, in terms of, actually very similar in how you think about it for gene editing. So you have the Cas protein itself and you kind of create a guide on it, so it's an RNA molecule that's specific to the nucleic acid that you want the protein to locate and find.
So, let's run with the example of, say, tuberculosis. You look at tuberculosis genome and say there are some specific sequence that are found in that species that you are not going to typically find in other contaminating species or in human genome. So you can design a guide sequence that will only target a sequence that's specific for tuberculosis. You create that, combine it with the nucleases, and then what you can do is that if you have some samples, like blood or spit or urine depending on the disease, you can take that, do some sample prep.
For figuring out, you can use a detection method. What that does is that when the CRISPR protein that's been programmed with this guide finds the target (so let's say there was tuberculosis present in the sample), it's going to bind to it and then the typical thing that we'll do is cut it, and that cutting can normally be to usual CRISPR editing.
So what's really cool is the particular nucleases that we use also have what we call this trans or collateral cutting activity so kind of conditional, like having found their targets, having found tuberculosis, they'll actually do all this extra cutting of DNA molecules in the solution that aren't the target necessarily. What we do with that is we can leverage that as two things. One is that it's like an amplification. So you go from, say, cutting the single molecule to cutting multiple molecules.
And then the second part is to put these little recorder molecules in the solution that say when these recorder molecules are cut through that activity they light up so that you can read it through, like example fluorescence. So that's kind of what's going on on a molecular level.
Trevor: Yeah, so the two proteins right now are Cas12 and Cas13, so Cas12 for searching through DNA and Cas13 for RNA. And the way it works, in terms of, actually very similar in how you think about it for gene editing. So you have the Cas protein itself and you kind of create a guide on it, so it's an RNA molecule that's specific to the nucleic acid that you want the protein to locate and find.
So, let's run with the example of, say, tuberculosis. You look at tuberculosis genome and say there are some specific sequence that are found in that species that you are not going to typically find in other contaminating species or in human genome. So you can design a guide sequence that will only target a sequence that's specific for tuberculosis. You create that, combine it with the nucleases, and then what you can do is that if you have some samples, like blood or spit or urine depending on the disease, you can take that, do some sample prep.
For figuring out, you can use a detection method. What that does is that when the CRISPR protein that's been programmed with this guide finds the target (so let's say there was tuberculosis present in the sample), it's going to bind to it and then the typical thing that we'll do is cut it, and that cutting can normally be to usual CRISPR editing.
So what's really cool is the particular nucleases that we use also have what we call this trans or collateral cutting activity so kind of conditional, like having found their targets, having found tuberculosis, they'll actually do all this extra cutting of DNA molecules in the solution that aren't the target necessarily. What we do with that is we can leverage that as two things. One is that it's like an amplification. So you go from, say, cutting the single molecule to cutting multiple molecules.
And then the second part is to put these little recorder molecules in the solution that say when these recorder molecules are cut through that activity they light up so that you can read it through, like example fluorescence. So that's kind of what's going on on a molecular level.
Minu: Right. So basically what you have is that recognizing the target is the same as how it happens in a regular CRISPR system, but then because these nucleuses also cut DNA and RNA even without sequence complementarity, you can amplify the signal, right?Trevor: Yeah, conditional on it having found it's target - and that's the tricky part, it's a read out from that search functionality.
Trevor: Yeah, conditional on it having found it's target - and that's the tricky part, it's a read out from that search functionality.
Challenges in Current Diagnostic Medical Technology
Though the technology is amazing, it comes with its share of challenges. How do you improve technology that’s been firmly in place for so long?
Kevin: You kind of mentioned this previously, but a lot of the current diagnostic tests are using old technology, like not necessarily bad technology but old, you know 30 years old or more. What current challenges exist in using that kind of technology in tests these days and how do you think, with your new technology, you're able to address those?
Trevor: So like you mentioned, a lot of these technologies are really great, I mean there's a reason they've been in use for a long time. But there's also a lot of ways that they can be improved, and I think one of the corporate ones that we think about at Mammoth is really the accessibility and how democratized they are. So really get us back to, are these technologies where you have to get to a doctor's office or you have to wait a long time; maybe it's a long turnaround time to get the result, or maybe they're very expensive.
And these are all things that we want to help solve with our technology. Because it's pretty shocking. When you get into the medical field and see how things operate, in many cases it's almost like it's like when you went to Google and you open the back door and there's a supercomputer running in the back. It's just, there's a lot of barriers to get in molecular information that I don't think necessarily have to be there, technologically.
So, that's one aspect of it is kind of democratizing access to really accurate larger of information by leveraging technologies that don't require as expensive equipment or don't require as much trained technician work to get the results. So that's one aspect.
And the other aspect is that, we're really excited about the potential in a CRISPR system software just have extremely high access, you know relative to current systems; just really leveraging the power of that search functionality of the CRISPR protein to get really sensitive and specific results.
And this doesn't mean that it has to be one or the other. We’re really excited about, and think it’s a really important point to mention the potential of the combined CRISPR systems with existing systems to leverage the strengths of all these different technologies. And there's a lot of exciting ways of thinking about doing that as well.
Kevin: One other kind of unique area in some ways is that, some other tests like Elisa, you mentioned they focus on the proteins that are being made by cells or infectious agents. Your tests focus on the DNA or RNA, so are there some advantages to that approach?Trevor: I mean there are advantages to looking at all these different modules. I think the key thing there that we're excited about is that molecular methods for DNA and RNA have been a little bit less accessible we've seen over time. So we really wanted to make detection of DNA and RNA in a specific manner, and really democratize that and make that something that's not locked behind lots of financial doors or hoops that you have to go through. You know, like a doctor or a patient to have to actually get the results that are relevant. So I think it really comes down to that democratization aspect and making sure that if that's information that's critical to treatment, it can be accessed in a way that's more open and reasonable.
Trevor: I mean there are advantages to looking at all these different modules. I think the key thing there that we're excited about is that molecular methods for DNA and RNA have been a little bit less accessible we've seen over time. So we really wanted to make detection of DNA and RNA in a specific manner, and really democratize that and make that something that's not locked behind lots of financial doors or hoops that you have to go through. You know, like a doctor or a patient to have to actually get the results that are relevant. So I think it really comes down to that democratization aspect and making sure that if that's information that's critical to treatment, it can be accessed in a way that's more open and reasonable.
Potential Regulations for CRISPR Medical Diagnostics
CRISPR is a highly regulated branch of science, and has been from its very starts. Learn how this impacts the field of medical diagnostics.
Minu: In the past few years, CRISPR applications have been long anticipated and especially in healthcare, people have been hoping to have CRISPR-based trials which are now starting after going through a lot of regulation issues, and it’s the same in agriculture. Do you think that regulations for diagnostics would be easier?
Trevor: So, it's not a matter of easier it's just a different path and I think as a society we've decided that it's very important to us, for good reason, right? To have these regulatory processes that really make sure that something is very safe before it's actually used in a clinical setting. For therapeutics, there's one path, and for diagnostics, there's another, and we're actually really excited to go down that regulatory path because we're ready to prove our technology and show that we really can give extremely accurate and clinically-relevant results.
So we've fully embraced that regulatory process and to your point, it is different and it is on a different time scale than things on the therapeutic side, and I think that can be exciting to get products into consumers hands as quickly as possible. We are working to make sure that we are leveraging our technology and really bringing that into the clinic, in a responsible way, as fast as possible.
Minu: Safety is absolutely the main reason why we have all these regulations in place, but then thinking of any kind of home test for diagnosis, do you think there's going to be a downside to that in the sense of any false positives or panic based on results that you see at home without having an expert to interpret them for you? Or have you thought about that side already?Trevor: That's a great question and we don't want these diagnostics to go in a vacuum. Really our goal at Mammoth is for these to be an integrated part of the entire care system, right? And that's why we're excited, as part of, for example, our efforts on the home care side to really integrate these tightly with the existing care structure; whether that's telemedicine so you can connect with a trusted doctor, whether that's connecting you to physical locations that can then maybe prescribe a therapeutic that you can pick up, or something like that. We're definitely very invested in making sure that this is information that is clinically useful, and in the future, this is information with a very clear next step.
And there’s no ambiguity around “what does this result mean?” We believe that providing diagnostic results is only kind of half of the equation. The other half is making sure it's very clear what actions - or non-actions - should be taken because of that, especially in a home environment.
Trevor: That's a great question and we don't want these diagnostics to go in a vacuum. Really our goal at Mammoth is for these to be an integrated part of the entire care system, right? And that's why we're excited, as part of, for example, our efforts on the home care side to really integrate these tightly with the existing care structure; whether that's telemedicine so you can connect with a trusted doctor, whether that's connecting you to physical locations that can then maybe prescribe a therapeutic that you can pick up, or something like that. We're definitely very invested in making sure that this is information that is clinically useful, and in the future, this is information with a very clear next step.
And there’s no ambiguity around “what does this result mean?” We believe that providing diagnostic results is only kind of half of the equation. The other half is making sure it's very clear what actions - or non-actions - should be taken because of that, especially in a home environment.
When Can We Buy CRISPR Diagnostics Tests at Walmart?
Mammoth is aiming to put diagnostic tests in the palm of your hand. Learn how this could work, and when it will be available to the average consumer.
Kevin: One of your main missions is to empower people to take a larger part in their healthcare, which means they can more easily access clinical tests. How do you envision it actually working? Would these tests be ordered by a doctor and then shipped to individuals or would it be even easier to go over the counter? For example, stop by Target and buy a test to see if you have influenza or TB or anything else?Trevor: That's really going to depend on the indication, but our long-term position is that you could go to a Walgreens or a CVS or a Walmart or any of these other kinds of neighborhood locations where you might pick up these types of items and get an off-the-shelf test for some diseases, whether that's some sort of respiratory or another disease. I think that's still to be determined in terms of what's useful at home. But really that is the long-term goal is that for things where it makes sense clinically. There would be accessibility and democratization of the access to that molecular information.Trevor: That's really going to depend on the indication, but our long-term position is that you could go to a Walgreens or a CVS or a Walmart or any of these other kinds of neighborhood locations where you might pick up these types of items and get an off-the-shelf test for some diseases, whether that's some sort of respiratory or another disease. I think that's still to be determined in terms of what's useful at home. But really that is the long-term goal is that for things where it makes sense clinically. There would be accessibility and democratization of the access to that molecular information.
Trevor: That's really going to depend on the indication, but our long-term position is that you could go to a Walgreens or a CVS or a Walmart or any of these other kinds of neighborhood locations where you might pick up these types of items and get an off-the-shelf test for some diseases, whether that's some sort of respiratory or another disease. I think that's still to be determined in terms of what's useful at home. But really that is the long-term goal is that for things where it makes sense clinically. There would be accessibility and democratization of the access to that molecular information.Trevor: That's really going to depend on the indication, but our long-term position is that you could go to a Walgreens or a CVS or a Walmart or any of these other kinds of neighborhood locations where you might pick up these types of items and get an off-the-shelf test for some diseases, whether that's some sort of respiratory or another disease. I think that's still to be determined in terms of what's useful at home. But really that is the long-term goal is that for things where it makes sense clinically. There would be accessibility and democratization of the access to that molecular information.
Kevin: I think it still being worked out which tests would be available at that level, but can you speculate on how far away you think we are from having these CRISPR-based tests available to acquire over the counter?Trevor: Yeah, so that part we're definitely excited about moving there as quickly as possible. I think it's premature to state an exact timeline on when that type of accessibility will be available, but that's definitely our vision for where this is headed. I think it’s something that's possible in the near future; it's not something that's ten years away in my opinion, but I don't think that it's prudent to say it’s coming as early as three years away. I think that there's definitely work to be done there and it’s a direction we're moving more towards every day.
Trevor: Yeah, so that part we're definitely excited about moving there as quickly as possible. I think it's premature to state an exact timeline on when that type of accessibility will be available, but that's definitely our vision for where this is headed. I think it’s something that's possible in the near future; it's not something that's ten years away in my opinion, but I don't think that it's prudent to say it’s coming as early as three years away. I think that there's definitely work to be done there and it’s a direction we're moving more towards every day.
Kevin: One other thing I don't really think we touched on yet is that the test might be read out on sort an app or a smartphone. Can you talk more about how that part of it will work?Trevor: There's a lot of different ways that you might utilize this type of testing, but we’re excited about the potential of leveraging that supercomputer that you have in your pocket as a tool that could actually be used in the diagnostic realm. And what's exciting about that is that means it's even more accessible, because you don't need to buy some sort of reader that's specifically for diagnostics only. It's just leveraging a reader and a computer that's in your pocket to give you more information. It really comes down to the accessibility, and we're excited to leverage that existing infrastructure in your home and in your pocket to deliver these results.
Trevor: There's a lot of different ways that you might utilize this type of testing, but we’re excited about the potential of leveraging that supercomputer that you have in your pocket as a tool that could actually be used in the diagnostic realm. And what's exciting about that is that means it's even more accessible, because you don't need to buy some sort of reader that's specifically for diagnostics only. It's just leveraging a reader and a computer that's in your pocket to give you more information. It really comes down to the accessibility, and we're excited to leverage that existing infrastructure in your home and in your pocket to deliver these results.
Kevin: So more specifically, how would it work? Would it be like you take a picture of the test? I understand it's small, roughly credit card-sized, essentially like a piece of paper that is the test. Would you take a picture of it and then an app reads out the signal?Trevor: So our vision for how it would work is that one format could be used as a paper-based vision, but we also imagine a lot of different other formats. I think if we run with the paper-based format, it would be as simple as taking a picture and we email the results. And that can help you standardize things, make sure that you're reading it correctly. It can also make sure that you're connecting that result with seeing your physician so they can get that data and they can help you think through next steps.
So it kind of serves two purposes: one is, making sure that you read the result in a very standardized way, and the second one is making sure that you're connected to the rest of the chain of care and getting the right information for the next step.
Trevor: So our vision for how it would work is that one format could be used as a paper-based vision, but we also imagine a lot of different other formats. I think if we run with the paper-based format, it would be as simple as taking a picture and we email the results. And that can help you standardize things, make sure that you're reading it correctly. It can also make sure that you're connecting that result with seeing your physician so they can get that data and they can help you think through next steps.
So it kind of serves two purposes: one is, making sure that you read the result in a very standardized way, and the second one is making sure that you're connected to the rest of the chain of care and getting the right information for the next step.
Kevin: So we've talked mostly about its application in healthcare, but your technology's not limited to that space. Can you tell us a little bit more about other areas you'd think you might be able to impact?Trevor: That's the other exciting part is there are just so many new areas in our lives and in the society that really rely on diagnostics when you get down to it. Whether that's, in healthcare - that's one that personally matters to us a lot - but outside of that, you can imagine things in agriculture, forensics, industry looking at various kind of factories, water-pipe lines, pollution avoided items, and so on. And then, of course, thinking more broadly, when we talk about healthcare we're usually talking about the United States but I think this technology could be incredibly impactful in the developing world, in countries that really don't have access at all to this type of molecular information. I mean I think that's going to be incredibly transformative to be able to get these tools in the hands of the people that didn't have any way to access this information before.
Trevor: That's the other exciting part is there are just so many new areas in our lives and in the society that really rely on diagnostics when you get down to it. Whether that's, in healthcare - that's one that personally matters to us a lot - but outside of that, you can imagine things in agriculture, forensics, industry looking at various kind of factories, water-pipe lines, pollution avoided items, and so on. And then, of course, thinking more broadly, when we talk about healthcare we're usually talking about the United States but I think this technology could be incredibly impactful in the developing world, in countries that really don't have access at all to this type of molecular information. I mean I think that's going to be incredibly transformative to be able to get these tools in the hands of the people that didn't have any way to access this information before.
SHERLOCK CRISPR Kit: The Next Generation of Diagnostics (with Podcast)
CRISPR is a novel gene-editing tool that is used for both therapeutic and diagnostic applications. Companies like Sherlock Biosciences, are using CRISPR to develop diagnostic tests to detect major diseases. Omar Abudayyeh and Jonathan Gootenberg, inventors of SHERLOCK––a CRISPR diagnostic kit, get candid about their journey from the lab to starting their company.

The Future of CRISPR Diagnostics
What else is to come for the future of this revolutionary technology? What other ways can it be applied in our world, and who will benefit from it most?
Minu: I think this tool is very useful for diagnostics and for many other purposes as you said, and it's interesting to consider that CRISPR basically just came out about five years ago and it has already reached this level. So could you speculate what you think the CRISPR situation will be five years from now?Trevor: Yeah, I mean it's tough, right? Because it's probably going to exceed whatever expectations you put on it. So, I think five years from now, I would really hope that we're in a place where there have been diagnostic and therapeutic uses of CRISPR that are actually helping people day-to-day. I think that would be super transformative in terms of having a healthcare system that serves people better, helping them live healthier and longer lives. So that's kind of the base level of what I would hope would be accomplished.
Beyond that though, I think we're gonna find more and more interesting uses that we haven't even thought of yet. I think people wouldn't have expected that you could use CRISPR in this way for diagnostics; that came out of nowhere when Jennifer's lab invented this and I think there'll be further inventions like that where it's just like “Wow, I had no idea that CRISPR could be used that way!” And I think that's what's exciting about the field, and that's why at Mammoth we've really broadened our thinking about CRISPR to be ‘CRISPR as search engine’ and not necessarily CRISPR as editor or CRISPR as some sort of readout. We see CRISPR as biology's google. And that's why we don't want to circumscribe CRISPR to just have one application, because I think it really has potential in many many areas.
Trevor: Yeah, I mean it's tough, right? Because it's probably going to exceed whatever expectations you put on it. So, I think five years from now, I would really hope that we're in a place where there have been diagnostic and therapeutic uses of CRISPR that are actually helping people day-to-day. I think that would be super transformative in terms of having a healthcare system that serves people better, helping them live healthier and longer lives. So that's kind of the base level of what I would hope would be accomplished.
Beyond that though, I think we're gonna find more and more interesting uses that we haven't even thought of yet. I think people wouldn't have expected that you could use CRISPR in this way for diagnostics; that came out of nowhere when Jennifer's lab invented this and I think there'll be further inventions like that where it's just like “Wow, I had no idea that CRISPR could be used that way!” And I think that's what's exciting about the field, and that's why at Mammoth we've really broadened our thinking about CRISPR to be ‘CRISPR as search engine’ and not necessarily CRISPR as editor or CRISPR as some sort of readout. We see CRISPR as biology's google. And that's why we don't want to circumscribe CRISPR to just have one application, because I think it really has potential in many many areas.
Minu: And so one fun question in the end which I've been very curious about myself is how did you settle on the name Mammoth?Trevor: Yeah, I wish I had a better answer than I do. So originally we were called Apheleia from, I think, the Greek goddess of simplicity of medicine or something like that - I’d have to look it up, but that name's pretty hard to spell! If you want to try spelling it right now, maybe you're better at spelling than I am, but a bit of a rough one to try to spell when you’re typing in a URL, so we changed the name. And it's funny, one of the first things one of our original investors told us is we have to change the name, this is not going to work. They rated the name like a one or two out of ten on their scale so we were decided to change the name.
We started brainstorming, came up with huge lists of names, a bunch of stuff. And we had this idea about animals and mammoth was one of them and, I don't know, we just looked at it like, yeah, that makes sense. And of course, thinking about this very large animal, like the elephant in the room that you can't ignore, but it’s the mammoth you can’t ignore. And also, you know, a bit of a cheeky play on this idea of CRISPR mammoths. So it works on a lot of levels, and mammoths are really cool so we decided to be represented by a mammoth.
Trevor: Yeah, I wish I had a better answer than I do. So originally we were called Apheleia from, I think, the Greek goddess of simplicity of medicine or something like that - I’d have to look it up, but that name's pretty hard to spell! If you want to try spelling it right now, maybe you're better at spelling than I am, but a bit of a rough one to try to spell when you’re typing in a URL, so we changed the name. And it's funny, one of the first things one of our original investors told us is we have to change the name, this is not going to work. They rated the name like a one or two out of ten on their scale so we were decided to change the name.
We started brainstorming, came up with huge lists of names, a bunch of stuff. And we had this idea about animals and mammoth was one of them and, I don't know, we just looked at it like, yeah, that makes sense. And of course, thinking about this very large animal, like the elephant in the room that you can't ignore, but it’s the mammoth you can’t ignore. And also, you know, a bit of a cheeky play on this idea of CRISPR mammoths. So it works on a lot of levels, and mammoths are really cool so we decided to be represented by a mammoth.
Minu: Yeah I was wondering if there was any connection to the fact that, based on the project of trying to bring back the wooly mammoth and maybe you thought, yeah that's the use potential of CRISPR.
Trevor: Yeah, there you go there's another CRISPR application right in the name!
Did you enjoy this episode and would love to stay informed about the latest CRISPR trends? subscribe to CRISPR Cuts on iTunes. If you want to get in touch with Martin directly, you can email him at [email protected] or follow him on Twitter at martintrevor_.
Trevor: Yeah, there you go there's another CRISPR application right in the name!
Did you enjoy this episode and would love to stay informed about the latest CRISPR trends? subscribe to CRISPR Cuts on iTunes. If you want to get in touch with Martin directly, you can email him at [email protected] or follow him on Twitter at martintrevor_.
Other CRISPR Podcasts That You Might Enjoy
Check out these podcasts on various topics of CRISPR science to get acquainted with the latest news, and hear about the technology straight from the mouths of the prominent CRISPR scientists in the field.
Andrew Porterfield | CRISPR in the Grocery Store
Learn more about CRISPR and GMOs, how CRISPR actually modifies organisms to help in the world of agriculture, and what’s coming to a store near you.
Jennifer Doudna | Intelligent Redesign
From Doudna herself, learn about the profound implications of her discovery, and what it means for our future world.
Ania Wronski | How Knockout Cell Pools are Revolutionizing CRISPR Research
For an in-depth look at the science of CRISPR, learn more about knockout cell pools, how they work, and why they’ve changed CRISPR forever.
Reed Kelso | Enabling CRISPR Access for All Scientists
Learn more about what Synthego is doing to make CRISPR more accessible to scientists from Synthego’s own Head of Cell Engineering Research and Applications.
Radiolab | Update: CRISPR
In this update on what’s been going on in the world of CRISPR, learn about the latest breakthroughs from a variety of scientists and special guests.
Alison van Eenennaam | Editing Cattle Using CRISPR
Learn how scientist Alison van Eenennaam is using CRISPR to help make cattle disease-resistant, and how CRISPR can help save the cattle-raising industry.
If you want to find other great podcasts to keep learning about CRISPR and science in general, check out our list of the top CRISPR podcasts and the best science podcasts to find more great episodes to stay up-to-date with the latest developments. If you prefer other ways to get your CRISPR news, head over to our comprehensive list of all the best CRISPR news sources to find the best option for your favorite way to learn.