The CRISPR field is moving fast! Don’t worry, we’ve got you covered. Check in every week for a quick summary of the biggest news and developments in genome engineering research so you can stay up to date with what’s happening in the world of CRISPR.
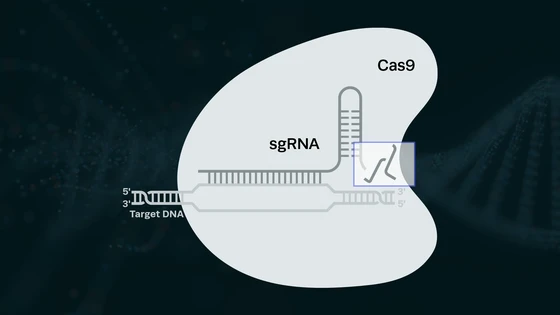
This week we report on a new study published in Nature Genetics by researchers at the University of California, Berkeley. The report sheds light on how DNA is repaired after the Cas9 nuclease makes a double-strand break (DSB). It is generally understood that this DSBs can be repaired by two different pathways: non-homologous end joining (NHEJ) or homology-directed repair (HDR). NHEJ is often referred to as a “quick and dirty” error-prone procedure that randomly generates insertions or deletions (indels), while HDR is the preferred option for precise gene editing and is the mechanism used to generate a knock-in. The exact details of the underlying mechanisms for both repair pathways have not yet been completely elucidated. This study reveals the involvement of an unexpected protein in homology-directed repair.
How Do Cells Decide Between NHEJ & HDR to Repair CRISPR Cuts?
The researchers set out to better understand the NHEJ and HDR pathways. They were particularly interested in circumstances when one pathway predominates over the other. They used CRISPR to knock out more than 2000 genes (one at a time!) that are known or suspected to influence DNA repair. The Fanconi anemia group D2 protein, encoded by the FANCD2 gene, turned out to be the magic bullet that the team was looking for.
FANCD2 is involved in the Fanconi anemia pathway, which involves 21 separate proteins. If any of the genes encoding those 21 proteins are mutated, individuals develop a condition called Fanconi anemia. This is a rare inherited blood disorder that prevents the bone marrow from making enough new blood cells. Patients suffering from this disorder often experience extreme fatigue, frequent infections, and problems with blood clotting. Fanconi anemia is also associated with birth defects and an elevated risk of cancer.
Fanconi Anemia Pathway Is Crucial For CRISPR-Induced Precision HDR Repair
“The Fanconi anemia pathway does not directly impact error-prone, non-homologous end joining, but instead diverts repair toward single-strand template repair,” Dr. Chris D. Richardson, first author of the study, wrote in the manuscript. “Furthermore, FANCD2 protein localizes to Cas9-induced double-strand breaks, indicating a direct role in regulating genome editing.”
Knowledge of a link between CRISPR editing repair and the Fanconi anemia pathway throws planned CRISPR treatments for the treatment of Fanconi anemia itself into question. If the Fanconi anemia pathway is defective, which is the case in Fanconi anemia patients by definition, then dysfunctional genes are unlikely to be successfully replaced with working genes after Cas9 makes a double-strand break if those same gene products are also required for HDR.
The study provides new useful and important information to ensure efficiency and precision when modifying genes using CRISPR-Cas9 technology. This research may help develop methods for effectively replacing genes in different cell types in the future.
More CRISPR in the News…
In Synthego News: Synthego Genome Engineering SolutionsIn Synthego News: Synthego Genome Engineering Solutions
The wonderful team at Genetic Engineering & Biotechnology News have crowned the Synthego website as this issue’s “Best of the Web” – hoorah!
In Synthego News: Synthego Genome Engineering Solutions
The wonderful team at Genetic Engineering & Biotechnology News have crowned the Synthego website as this issue’s “Best of the Web” – hoorah!
In Using CRISPR to Drive Evolution: CRISPR diversifies: Cut, paste, on, off, and now– evolve!
Evolution relies on the accumulation of random mutations in the genome that are passed to future generations, with specific mutations becoming more prevalent in a population if they offer an advantage to the organism. If researchers could re-create evolution in the lab, it would potentially enable creating novel beneficial functionalities in organisms. Scientists have turned to CRISPR in an attempt to accelerate evolution in vitro. A team of researchers at the Innovative Genomics Institute have developed a new system, EvolvR, that uses engineered DNA polymerases and CRISPR-guided nickases that cause a single-strand cut in the genome. Their tool induces a series of random mutations in DNA fragments of up to 350 base pairs. Fine-tuning this method could open up a vast set of applications in biotechnology in the future.
In Angelman Syndrome Research: Angelman Syndrome Foundation awards $200,000 to research CRISPR as potential gene therapy
Angelman syndrome is a rare congenital disorder caused by loss of function of the UBE3A gene that affects an estimated 1 in 12,000 to 20,000 people. The disorder is characterized by delayed development, intellectual disability, severe speech impairment, and problems with movement and balance. The Angelman Syndrome Foundation recently awarded $200,000 research grant to explore the potential for CRISPR-Cas9 as a therapy to return functionality to the faulty UBE3A gene.
In CRISPR Legal Decisions: A European Union court has issued a new decision about GMOs
The Court of Justice of the European Union recently announced that plants with modified genomes as a result of CRISPR technology are subject to the EU’s strict regulations on GM crops. They stated that “Organisms obtained by mutagenesis are GMOs and are, in principle, subject to the obligations laid down by the GMO Directive.” The US Department of Agriculture commented on the decision, with Agriculture Secretary Sonny Perdue saying, “Government policies should encourage scientific innovation without creating unnecessary barriers or unjustifiably stigmatizing technologies” in a press release shortly after the ruling.
In Inner Ear Research: Fibroblast growth factor 12 is expressed in spiral and vestibular ganglia and necessary for auditory and equilibrium function
Researchers from Japan have uncovered the localization and function of fibroblast growth factor 12 (FGF12) as a transcript enriched in the inner ear. They used CRISPR-Cas9 technology to investigate FGF12 function using FGF12-knockout mice, and demonstrated that the growth factor is needed not only for normal hearing abilities but also for sufficient special awareness and balance.
Cell Engineering 101
CRISPR has ignited a revolution. Although it’s a relatively recent discovery in the history of biotechnology, CRISPR has quickly become a standard laboratory tool and cell engineering is transforming research. One of the most widely used applications of CRISPR is knocking out specific genes in cell lines to interrogate gene function.