Contents
Like many areas of biomedical research, CRISPR-Cas9 technology has revolutionized the field of xenotransplantation. By enabling multiple, high-efficiency gene edits in animals like pigs, CRISPR has overcome some of the key obstacles in this field, pushing xenotransplants into the clinic - and the global spotlight.
Recent breakthroughs in xenotransplantation, including the first animal-to-human heart transplant, have caused a lot of excitement in the scientific community and the general public alike. In this CRISPR Conversation, we feature xenotransplantation trailblazer Dr. Björn Petersen, of the Institute of Farm Animal Genetics at Friedrich-Loeffler-Institut in Germany.
Björn’s research group has pioneered the use of CRISPR to modify pig genomes, minimizing adverse reactions for successful organ transplantation. Learn more about Björn’s work and his thoughts on the recent progress in the xenotransplantation field in this extended interview.
Pig Organ Xenotransplant Makes it into the Clinic After Decades of Research
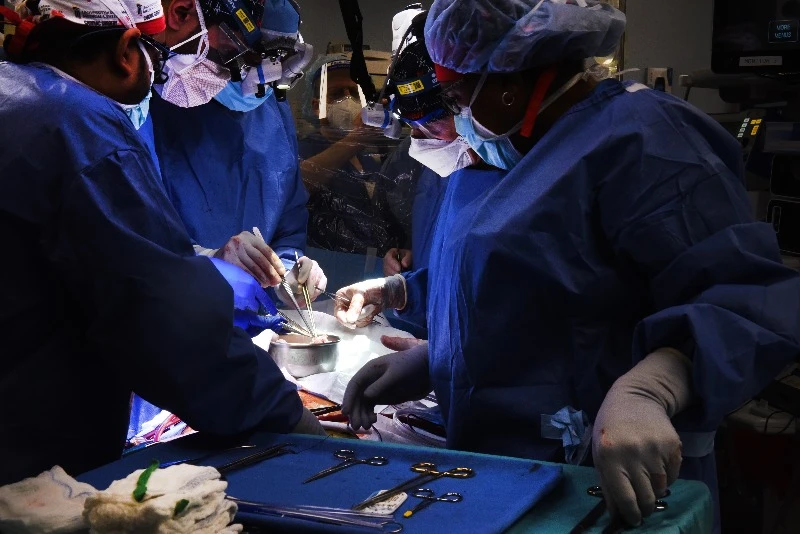
A key news story early in 2022 was the first successful xenotransplant. Researchers from The University of Maryland Medical School harvested a heart from a gene-edited pig and transplanted it into a human patient. The importance of this breakthrough cannot be understated, as Björn elaborates.
Björn: I've been working for more than 20 years in the xenotransplantation field. For many years, the German consortium and the group of Revivicor, who now are producers of the pig heart that was transplanted, were the only two groups in the world that kept xenotransplantation ‘alive’. So this is a huge step forward, and it's the first time that a pig heart was transplanted into a human body. Xenotransplantation is really reaching the clinical stage now, and that is fantastic!
Key Challenges in Xenotransplant Research
Xenotransplantation has been a topic of research for decades, but scientists working in this field have faced several seemingly insurmountable obstacles. As a veteran in the xenotransplant field, Björn explains the major hurdles that have prevented animal organs from being used in human patients until recently.
What are the major challenges associated with xenotransplantation?Björn: The problem is that the pigs and humans are quite distinct from each other and there are many epitopes that cause very severe immunological reactions. The first one is the sugar molecule, which is a galactose epitope; it's a major xenoantigen. Humans have lost that gene during evolution but have been exposed to the molecule through contact with bacteria. So we have antibodies in our blood flow directed against this sugar molecule - that means that if you transplant a native pig heart, it would immediately be destroyed. This is called the hyperacute rejection response. There are two other known epitopes that cause similar, but slightly reduced, reactions from the human immune system.
So the first step is to overcome these immune reactions. And to do that, you have to modify the pig genome. For a long time, we did not have the right tools. That changed with the development of genome engineering tools such as zinc finger nucleases, then TALENs, and now the CRISPR-Cas system.
The second step is that you have some incompatibilities between physiological parameters, like the coagulation system and some other things. So you have to overcome these incompatibilities as well.
The next thing is the size of the pig heart. Usually, a pig that is slaughtered weighs about 115 kilograms, but they can become much larger, up to 400 kilograms. That means another critical factor is to reduce the size of the pigs.
Porcine endogenous retroviruses (PERVs) are also considered to be a potential threat. eGenesis, which is another company that is working on xenotransplantation, use a CRISPR-Cas system to make the PERVs in the pig genome non-functional.
So you have to perform a lot of editing of the pig genome to make it transplantable into a human body, and that is a great challenge. And for many years, we had to figure out the critical factors are and explore each hurdle. That’s why it took so long to now enter the clinical stage.
Björn: The problem is that the pigs and humans are quite distinct from each other and there are many epitopes that cause very severe immunological reactions. The first one is the sugar molecule, which is a galactose epitope; it's a major xenoantigen. Humans have lost that gene during evolution but have been exposed to the molecule through contact with bacteria. So we have antibodies in our blood flow directed against this sugar molecule - that means that if you transplant a native pig heart, it would immediately be destroyed. This is called the hyperacute rejection response. There are two other known epitopes that cause similar, but slightly reduced, reactions from the human immune system.
So the first step is to overcome these immune reactions. And to do that, you have to modify the pig genome. For a long time, we did not have the right tools. That changed with the development of genome engineering tools such as zinc finger nucleases, then TALENs, and now the CRISPR-Cas system.
The second step is that you have some incompatibilities between physiological parameters, like the coagulation system and some other things. So you have to overcome these incompatibilities as well.
The next thing is the size of the pig heart. Usually, a pig that is slaughtered weighs about 115 kilograms, but they can become much larger, up to 400 kilograms. That means another critical factor is to reduce the size of the pigs.
Porcine endogenous retroviruses (PERVs) are also considered to be a potential threat. eGenesis, which is another company that is working on xenotransplantation, use a CRISPR-Cas system to make the PERVs in the pig genome non-functional.
So you have to perform a lot of editing of the pig genome to make it transplantable into a human body, and that is a great challenge. And for many years, we had to figure out the critical factors are and explore each hurdle. That’s why it took so long to now enter the clinical stage.
You mentioned the size of the pig is a critical factor. What strategies do you use to address this problem?Björn: There are several ways you could solve the problem. The first one is you could harvest the organ when the pig is younger. The problem with that is, if you transplant a pig heart into a normal adult of about 75 kilograms, you have to take a pig heart that comes from a pig of an age that is maybe three months old. But at that time point, the pig heart might not be fully matured.
What we observed in transplantations of pig hearts into baboons was some hypertrophy of the pig heart. This could be because the blood pressure of a primate is much higher than the blood pressure in pigs. And if you have an immature heart, this can lead to dilatation and then hypertrophy.
The other option is to reduce the size, then you can use older pigs. For example, you can knock out a specific gene, which encodes for the growth hormone receptor. If you do that, the pig only reaches about 75 to 100 kilograms, which is quite similar to humans. So then you can use the heart from a fully grown pig, which is completely mature. This is one of the best options, so that was done in the recent heart xenotransplantation.
You can also use pig breeds that are smaller, but the problem with the mini pigs is in most cases, they contain a lot of PERVs. There are only some mini pig breeds that are almost free, at least of some subtypes of PERVs. For example, the Auckland mini pigs could be another source for xenotransplantation in the near future because they are free of the PERV-C subtype, which is capable of recombination.
Björn: There are several ways you could solve the problem. The first one is you could harvest the organ when the pig is younger. The problem with that is, if you transplant a pig heart into a normal adult of about 75 kilograms, you have to take a pig heart that comes from a pig of an age that is maybe three months old. But at that time point, the pig heart might not be fully matured.
What we observed in transplantations of pig hearts into baboons was some hypertrophy of the pig heart. This could be because the blood pressure of a primate is much higher than the blood pressure in pigs. And if you have an immature heart, this can lead to dilatation and then hypertrophy.
The other option is to reduce the size, then you can use older pigs. For example, you can knock out a specific gene, which encodes for the growth hormone receptor. If you do that, the pig only reaches about 75 to 100 kilograms, which is quite similar to humans. So then you can use the heart from a fully grown pig, which is completely mature. This is one of the best options, so that was done in the recent heart xenotransplantation.
You can also use pig breeds that are smaller, but the problem with the mini pigs is in most cases, they contain a lot of PERVs. There are only some mini pig breeds that are almost free, at least of some subtypes of PERVs. For example, the Auckland mini pigs could be another source for xenotransplantation in the near future because they are free of the PERV-C subtype, which is capable of recombination.
And are PERVs a major issue for pig xenotransplants?Björn: We have some virologists in our xenotransplantation consortium, and they do not really think that most PERVs are a real threat to xenotransplantation. In vitro, PERVs are capable to infect human cells but it never happened in vivo.
In the Czech Republic, there have been pig skin transplants and there's never been any observed infection of human patients with PERVs. There are also commercial pig breeds that are free of PERV-C, so it should not be a big issue.
Björn: We have some virologists in our xenotransplantation consortium, and they do not really think that most PERVs are a real threat to xenotransplantation. In vitro, PERVs are capable to infect human cells but it never happened in vivo.
In the Czech Republic, there have been pig skin transplants and there's never been any observed infection of human patients with PERVs. There are also commercial pig breeds that are free of PERV-C, so it should not be a big issue.
CRISPR-Cas9: A Major Breakthrough for Xenotransplants

Since the advent of CRISPR-Cas9 gene editing in 2012, progress in the xenotransplant field has come in leaps and bounds. Björn describes the huge impact CRISPR has made in his own research and in the xenotransplant field more broadly.
How has CRISPR technology helped solve the challenges in this field, and how has it helped push xenotransplants into a clinical setting?Björn: CRISPR has revolutionized the field of genome editing in farm animals and it’s a breakthrough technology for xenotransplantation. Before CRISPR, you had to construct knockout vectors and rely on homologous recombination with an efficiency of 1:1000000 cells carrying the desired knockout. When you think about the removal of PERVs, we could not achieve the results that were published by eGenesis by using ZFNs or TALENs. CRISPR is much easier to use, much faster, more efficient, and much cheaper. Importantly, you can perform multiple edits.
Björn: CRISPR has revolutionized the field of genome editing in farm animals and it’s a breakthrough technology for xenotransplantation. Before CRISPR, you had to construct knockout vectors and rely on homologous recombination with an efficiency of 1:1000000 cells carrying the desired knockout. When you think about the removal of PERVs, we could not achieve the results that were published by eGenesis by using ZFNs or TALENs. CRISPR is much easier to use, much faster, more efficient, and much cheaper. Importantly, you can perform multiple edits.
"So the invention of the CRISPR-Cas system has brought a lot of companies back into the field of xenotransplantation because they saw the possibility to achieve all these genetic modifications that are necessary to provide a pig organ that might be suitable for human transplantation. That brought in a lot of money back into the business and sped up the whole process. And that would not have been feasible without CRISPR-Cas. "
Tell us more about the genes that underpin the immune rejection of xenografts.Björn: There are three main genes that need to be knocked out, and they are all sugar molecules. There’s the GGTA1 gene, which encodes the galactose epitope. And then there is a Neu5Gc epitope, which is encoded by the CMAH gene. And the third one is the product of the gene called B4GALNT2. The first two are not expressed in humans. And the third one is expressed in humans, but in different locations compared to the pig. The fourth gene knockout would be to reduce the size. The fifth could be to maybe knock out some PERVs. So you need at least 3, but up to 5, gene knockouts for a heart xenotransplant.
Björn: There are three main genes that need to be knocked out, and they are all sugar molecules. There’s the GGTA1 gene, which encodes the galactose epitope. And then there is a Neu5Gc epitope, which is encoded by the CMAH gene. And the third one is the product of the gene called B4GALNT2. The first two are not expressed in humans. And the third one is expressed in humans, but in different locations compared to the pig. The fourth gene knockout would be to reduce the size. The fifth could be to maybe knock out some PERVs. So you need at least 3, but up to 5, gene knockouts for a heart xenotransplant.
Are there different genes involved in immune rejection in different organs?Björn: Yes, there are some specific responses you have to address in the different organs. For example, the heart is a quite simple organ - it's just a muscle pump, and there's not so much rejection response, and you don’t really have to overcome so many incompatibilities. This is much more difficult in the kidney, where you have some physiological processes that have to be well-orchestrated. It's completely different in the liver, which is quite difficult, and in the lung. The shortest survival of a xenotransplant in a baboon is with the lung; it seems to be a very difficult organ.
Björn: Yes, there are some specific responses you have to address in the different organs. For example, the heart is a quite simple organ - it's just a muscle pump, and there's not so much rejection response, and you don’t really have to overcome so many incompatibilities. This is much more difficult in the kidney, where you have some physiological processes that have to be well-orchestrated. It's completely different in the liver, which is quite difficult, and in the lung. The shortest survival of a xenotransplant in a baboon is with the lung; it seems to be a very difficult organ.
Is it necessary to perform CRISPR gene knock-ins, as well as knockouts, for xenotransplants?Björn: Yes, we have to knock in several [human] transgenes to overcome incompatibilities. For example, the pig heart that was recently transplanted was edited with CRISPR to have four gene knockouts and six transgenes. The difficulty with transgenes is that you have to put them all together at a specific locus in the pig genome. Otherwise, if you breed the animal, you would have complete segregation of the different transgenes. And that would make it very difficult to produce pigs by simple breeding. Then you would have to clone every single pig, which is very inefficient and laborious. So you have to really put them all together in the locus of the pig where you have permanent expression.
Björn: Yes, we have to knock in several [human] transgenes to overcome incompatibilities. For example, the pig heart that was recently transplanted was edited with CRISPR to have four gene knockouts and six transgenes. The difficulty with transgenes is that you have to put them all together at a specific locus in the pig genome. Otherwise, if you breed the animal, you would have complete segregation of the different transgenes. And that would make it very difficult to produce pigs by simple breeding. Then you would have to clone every single pig, which is very inefficient and laborious. So you have to really put them all together in the locus of the pig where you have permanent expression.
How has CRISPR technology helped in your own xenotransplant research, and how has Synthego helped?Björn: Our group is part of the DFG funded collaborative research center "Xenotransplantation". In this project, we are aiming to use pig organs as an alternative source for organ transplantation to fight the permanent shortage of available donor organs. To prevent rejection responses, the pig genome must be modified to abrogate specific epitopes on the pig organs that cause rejection responses.
We use CRISPR to target porcine genes that encode for xenoantigens and to modify the pig genome to mimic human disease mutations. We also try to find critical traits that confer resistance against specific pathogens. Other projects we are currently working on are a porcine hypertension model, sex determination in pigs and cattle, and disease resistance.
Björn: Our group is part of the DFG funded collaborative research center "Xenotransplantation". In this project, we are aiming to use pig organs as an alternative source for organ transplantation to fight the permanent shortage of available donor organs. To prevent rejection responses, the pig genome must be modified to abrogate specific epitopes on the pig organs that cause rejection responses.
We use CRISPR to target porcine genes that encode for xenoantigens and to modify the pig genome to mimic human disease mutations. We also try to find critical traits that confer resistance against specific pathogens. Other projects we are currently working on are a porcine hypertension model, sex determination in pigs and cattle, and disease resistance.
We were the first lab to publish a knockout of an endogenous pig gene by using ZFNs. When we saw the potential of the CRISPR-Cas system, we immediately tested it out and after that, we switched completely to CRISPR.
"We were one of the first laboratories that used CRISPR technology in xenotransplantation. We use Synthego’s synthetic sgRNA, which is essential for our research. Synthego ensures a high activity of their products, and the tools they provide are beneficial in achieving our goals. Synthego is not our only supplier, but it's one of our favorite companies. "
A Promising Future for Xenotransplantation
After the first successful xenotransplant into a human patient, we’re all wondering what the next step will be. Björn speculates on the future of the xenotransplant field, including potential refinements to gene editing and widespread use of this technology to save lives.
Now that CRISPR is being used to address the obstacles in this field, how far away are we from the widespread use of xenotransplants?Björn: It's hard to predict, but I think we're much closer now with this first heart xenotransplantation. So far, the patient has lived longer than the first human patient that received an allotransplant heart. It's a huge success, but it depends on the long-term results they achieve. Maybe they will find out that they have to perform some more genetic modifications, or something else will come up that could slow things down.
I would think we are maybe five to 10 years [from widespread use]. It takes some time to produce enough pigs for all these purposes, and the clinics must be trained to transplant the pig heart. For this recent heart transplant, they used an experimental immunosuppressive drug, so it depends if that also gets approved [by the FDA]. So there are still some questions, but that was the first step and it's a very important step.
Björn: It's hard to predict, but I think we're much closer now with this first heart xenotransplantation. So far, the patient has lived longer than the first human patient that received an allotransplant heart. It's a huge success, but it depends on the long-term results they achieve. Maybe they will find out that they have to perform some more genetic modifications, or something else will come up that could slow things down.
I would think we are maybe five to 10 years [from widespread use]. It takes some time to produce enough pigs for all these purposes, and the clinics must be trained to transplant the pig heart. For this recent heart transplant, they used an experimental immunosuppressive drug, so it depends if that also gets approved [by the FDA]. So there are still some questions, but that was the first step and it's a very important step.
What are your thoughts on the future of xenotransplantation, especially with the advances in CRISPR technology? Are more developments needed?Björn: We have all the technology in our hands now to reach our goal. Maybe there are some refinements necessary, based on the recent heart transplant - maybe they will have to add some more transgenes, or they will see now that one transgene is not expressed properly or has to be expressed at higher levels to have an effect. But these are all very important things that are moving xenotransplants closer to the clinic.
Björn: We have all the technology in our hands now to reach our goal. Maybe there are some refinements necessary, based on the recent heart transplant - maybe they will have to add some more transgenes, or they will see now that one transgene is not expressed properly or has to be expressed at higher levels to have an effect. But these are all very important things that are moving xenotransplants closer to the clinic.
Further Learning Resources
- Nature News: First pig-to-human heart transplant: what can scientists learn?
- CRISPR in cattle: Podcast with Dr. Alison van Eenennaam
- Blog: Transgenic animals
We hope this article got you excited about the future of xenotransplantation! Would you like to have your work featured on The Bench Blog? Simply fill out a few details in this form!