CRISPR Revolution in Cell & Gene therapy ebook
How CRISPR Gene Editing is Accelerating Cell & Gene Therapies

Kevin: Can you talk to us about your research and the work you are doing both as a clinician and as a research scientist?

Waseem: I am a clinician scientist, which means that I have trained in medicine, in pediatrics, but then diverged a little bit and spend most of my time in laboratory-based research. My work mostly focuses on translational aspects of immunology, in particular, in relation to bone marrow transplantation. Our hospital will look after large numbers of children requiring bone marrow transplantation for conditions such as leukemia, some blood disorders, and inherited defects of the immune system. Now, what that means is that over time, we have built quite a lot of expertise in being able to handle bone marrow and white blood cells, T-cells in particular for therapeutic purposes, and that's brought us into the arena of gene therapy and now most recently, gene editing.
Kevin: Can you tell us a little bit about the GOSH-- why it's a special place, its background and history, and how you came to be there?Waseem: So the hospital is a dedicated children's hospital and was one of the first of its kind. In the UK, our health service is supported by the National Health Service, which is very important because it means across the whole country, there's equality of access to patients for all kinds of conditions. As far as our particular group of patients is concerned, it is one of the two regional centers across the country that takes children for these rare immune deficiencies and so on.
I have actually been there since 1996, which is when I first worked as a senior house officer in a department, which at that time was called the Host Defense Unit, a grand term, but essentially dealt with all types of conditions where the immune system was either weak or needed repair, and that linked into transplantation, also infectious diseases. Over time, we built up expertise in the use of in particular viral vectors for engineering cells, repair of certain types of genetic disorders that are all conditions where you can harvest cells and manipulate them outside the body ex vivo in a cleanroom facility.
The hospital has some of the best GMP facilities in the country and has probably done the greatest number of different types of gene therapy, if not the greatest numbers of patients, and the different variety of conditions that we have treated has been quite remarkable.
Waseem: So the hospital is a dedicated children's hospital and was one of the first of its kind. In the UK, our health service is supported by the National Health Service, which is very important because it means across the whole country, there's equality of access to patients for all kinds of conditions. As far as our particular group of patients is concerned, it is one of the two regional centers across the country that takes children for these rare immune deficiencies and so on.
I have actually been there since 1996, which is when I first worked as a senior house officer in a department, which at that time was called the Host Defense Unit, a grand term, but essentially dealt with all types of conditions where the immune system was either weak or needed repair, and that linked into transplantation, also infectious diseases. Over time, we built up expertise in the use of in particular viral vectors for engineering cells, repair of certain types of genetic disorders that are all conditions where you can harvest cells and manipulate them outside the body ex vivo in a cleanroom facility.
The hospital has some of the best GMP facilities in the country and has probably done the greatest number of different types of gene therapy, if not the greatest numbers of patients, and the different variety of conditions that we have treated has been quite remarkable.
Kevin: Do you feel that seeing patients helps to really translate your lab work and kind of gives you a sense of purpose?Waseem: I do. I look after children coming in and out of transplantation. So I spend a day a week in that type of clinic and also trying to attend a number of multidisciplinary meetings, where there are different specialists in the room, designing patient plans and trying to work out the best options. That is actually critical for understanding where the unmet need is in terms of research, and we are sitting at the interface of what's known as translational research, grabbing things that are developing in the scientific arena, picking out the ones that we think will be useful and provide meaningful treatments in a reasonable time period and pushing them into early phase trials.
Now, if some of those treatments then turn out to be particularly useful or likely to have larger numbers of patients is a good route out into wider dissemination through drug companies and pharma and so on to pick up things and develop them into routine medicines.
Waseem: I do. I look after children coming in and out of transplantation. So I spend a day a week in that type of clinic and also trying to attend a number of multidisciplinary meetings, where there are different specialists in the room, designing patient plans and trying to work out the best options. That is actually critical for understanding where the unmet need is in terms of research, and we are sitting at the interface of what's known as translational research, grabbing things that are developing in the scientific arena, picking out the ones that we think will be useful and provide meaningful treatments in a reasonable time period and pushing them into early phase trials.
Now, if some of those treatments then turn out to be particularly useful or likely to have larger numbers of patients is a good route out into wider dissemination through drug companies and pharma and so on to pick up things and develop them into routine medicines.
Kevin: You mentioned some of your work developing vector-based gene therapies. Can you talk a little bit about how your lab has moved into utilizing CRISPR as a useful part of that?Waseem: So, our vector expertise is really interesting because all our understanding of how viral vectors work—we deal in particular with retroviral and lentiviral— has come from the enormous amount of effort from other groups that have studied how the biology of HIV reverse transcription in certain genes works. What we have managed to do is adapt and harness that understanding to develop therapies from the molecular biology and the life cycle of knowing how the viruses work, and then having appreciated how to try and use some of those tools, we initially became involved in some of the emerging gene editing technologies.
First, there was an iteration of tools called TALENs, and it has been around not for that long, probably since about 2010. I should say before that we were engaged in testing something called meganuclease and we in the laboratory were excited if we had 0.1% or 0.5% of cells that were edited. Then TALENs came along and suddenly the efficiency jumped up into the tens or twenties or fifties in terms of percentage of modification. Since 2012 or 2013, we have been developing tools derived from CRISPR, and indeed those themselves have evolved very rapidly, and even as we get ready to test them in the clinic, the versions that are now around and the techniques that are available now are improving on a weekly basis.
Kevin: That's great. Thanks for the interview, Waseem.
Waseem: So, our vector expertise is really interesting because all our understanding of how viral vectors work—we deal in particular with retroviral and lentiviral— has come from the enormous amount of effort from other groups that have studied how the biology of HIV reverse transcription in certain genes works. What we have managed to do is adapt and harness that understanding to develop therapies from the molecular biology and the life cycle of knowing how the viruses work, and then having appreciated how to try and use some of those tools, we initially became involved in some of the emerging gene editing technologies.
First, there was an iteration of tools called TALENs, and it has been around not for that long, probably since about 2010. I should say before that we were engaged in testing something called meganuclease and we in the laboratory were excited if we had 0.1% or 0.5% of cells that were edited. Then TALENs came along and suddenly the efficiency jumped up into the tens or twenties or fifties in terms of percentage of modification. Since 2012 or 2013, we have been developing tools derived from CRISPR, and indeed those themselves have evolved very rapidly, and even as we get ready to test them in the clinic, the versions that are now around and the techniques that are available now are improving on a weekly basis.
Kevin: That's great. Thanks for the interview, Waseem.
Kevin: Can you talk about how you first started interacting with our company around CRISPR, and how Synthego launching a GMP facility is going to help with your work?Waseem: Synthego has got an important track record in being able to supply in a very rapid manner guide RNAs, guide RNAs with relevant chemical modifications, and we have been getting those from you for some years now. We have been very pleased with the quality of the material that we have managed to use and test. Some of our projects are now in a translation or GMP phase. So, we need high-grade material that is being produced in a validated manner, and so that is what our next round of technical developments will be around.
Waseem: Synthego has got an important track record in being able to supply in a very rapid manner guide RNAs, guide RNAs with relevant chemical modifications, and we have been getting those from you for some years now. We have been very pleased with the quality of the material that we have managed to use and test. Some of our projects are now in a translation or GMP phase. So, we need high-grade material that is being produced in a validated manner, and so that is what our next round of technical developments will be around.
Kevin: Thinking back on some of your previous success stories, can you tell us a little bit about your involvement with the leukemia case with baby Layla back in 2015?
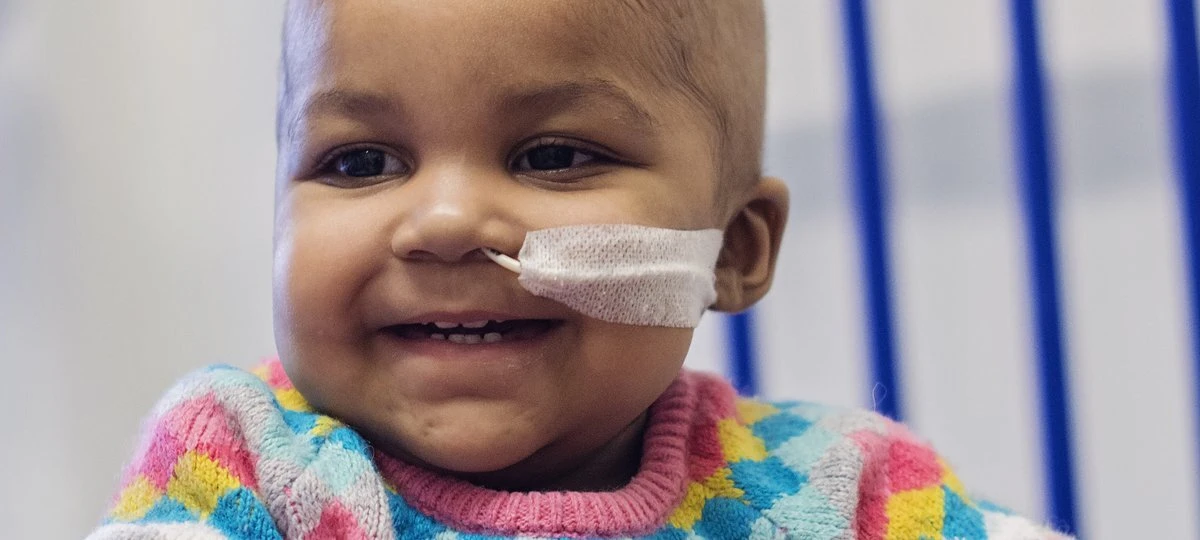
Image credit: World’s first use of gene-edited immune cells to treat ‘incurable’ leukemia, press release, GOSH.Waseem: We treated a couple of infants with a very difficult to manage relapsed refractory type of B-cell leukemia, and we engineered cells from unmatched healthy donor lymphocytes and engineered the cells to be devoid of T-cell receptor. We disarmed them to be devoid of another molecule called CD 52, and that allowed us to deliver a CAR therapy where otherwise neither patient had any real prospects of a therapeutic option, and both those treatments were successful, put the children in remission, and allowed them to proceed to bone marrow transplantation, and had good outcomes in both cases. So that set off multicenter studies in children and in adults, and those are still running and provided important proof of concept information to the field as a whole I think, that cells that are from an unmatched non-HLA matched donor can be used in an off-the-shelf way for these types of treatments.
Waseem: We treated a couple of infants with a very difficult to manage relapsed refractory type of B-cell leukemia, and we engineered cells from unmatched healthy donor lymphocytes and engineered the cells to be devoid of T-cell receptor. We disarmed them to be devoid of another molecule called CD 52, and that allowed us to deliver a CAR therapy where otherwise neither patient had any real prospects of a therapeutic option, and both those treatments were successful, put the children in remission, and allowed them to proceed to bone marrow transplantation, and had good outcomes in both cases. So that set off multicenter studies in children and in adults, and those are still running and provided important proof of concept information to the field as a whole I think, that cells that are from an unmatched non-HLA matched donor can be used in an off-the-shelf way for these types of treatments.
Kevin: Do you hope now that you can start to use CRISPR to develop a similar type of therapeutic approaches to help children out there?Waseem: So our next version is a CRISPR version where the guides are embedded in our lentiviral configuration, and then we have got follow up additional approaches such as multiplexed changes to the cells, which we think will improve the approach and widen the application to other types of disease.
Waseem: So our next version is a CRISPR version where the guides are embedded in our lentiviral configuration, and then we have got follow up additional approaches such as multiplexed changes to the cells, which we think will improve the approach and widen the application to other types of disease.