Over the last few decades, zebrafish has become increasingly popular as a model system for understanding vertebrate gene function as well for studying human genetic diseases. Since it’s recent discovery, CRISPR-Cas9, a groundbreaking technology, has quickly become a method of choice for genome engineering in zebrafish and other model organisms.
In this article, we will learn about zebrafish genetics, its role in CRISPR research, and why it has proven to be a useful model organism in advancing human genetic disease research. Specifically, we will cover:
Zebrafish: Why is it important for Disease Research?
Zebrafish (Danio rerio) is a small tropical, freshwater fish, that is found in South Asia. This tiny fish has been rapidly gaining popularity, along with other model organisms such as mice and yeast for modeling diseases. Mice, being mammals, are evolutionarily more similar to humans. However, zebrafish have several advantages over these furry creatures. Let us discuss some reasons that make this tiny fish an important model for studying human genetics and disease.
Unique characteristics of a zebrafish embryo
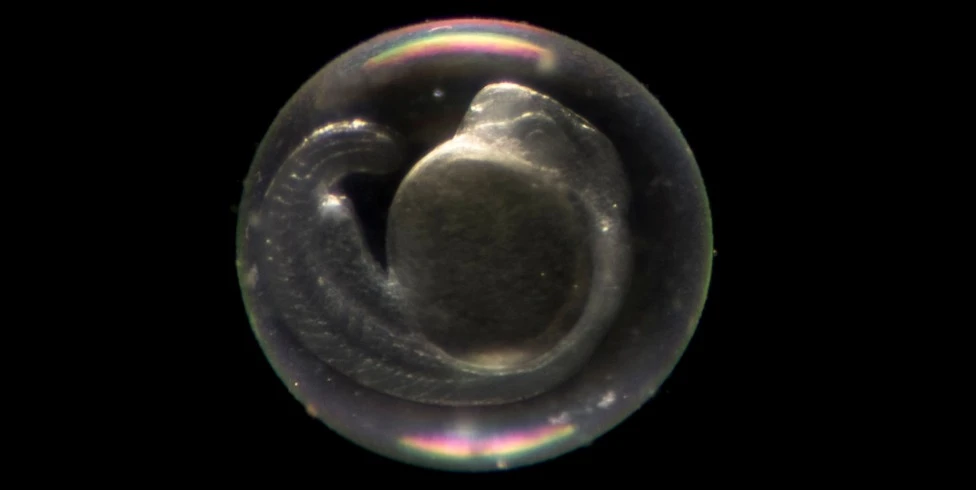
Zebrafish embryos are externally laid and fertilized, allowing scientists to easily manipulate them. During the early stages of development, fertilized eggs can be injected with DNA or RNA to modify their genetic makeup and generate knock-out or transgenic zebrafish. Scientists have also taken advantage of the fact that zebrafish embryos are transparent, enabling observation of growth and development under a microscope. In mice, this is not possible as embryos develop inside the mother.
Zebrafish genetics: Similarities between human & zebrafish genomeThe Sanger Institute initiated the zebrafish genome sequencing project in 2001. The sequencing of the entire genome of zebrafish revealed that 71.4 percent of human genes are found in zebrafish. Furthermore, 84 percent of genes known to be associated with human disease have a counterpart in zebrafish.
This similarity in genetic makeup with humans makes it easy to model diseases in zebrafish. By using zebrafish knockout or knock-in models, genes can be manipulated to understand disease pathways. For decades, targeted gene editing was not possible, limiting the ability of scientists to study individual genes. However, in the recent past, with the discovery of novel genetic engineering tools such as TALENs, ZFNs, and CRISPR-Cas9, researchers have overcome this challenge.
The Sanger Institute initiated the zebrafish genome sequencing project in 2001. The sequencing of the entire genome of zebrafish revealed that 71.4 percent of human genes are found in zebrafish. Furthermore, 84 percent of genes known to be associated with human disease have a counterpart in zebrafish.
This similarity in genetic makeup with humans makes it easy to model diseases in zebrafish. By using zebrafish knockout or knock-in models, genes can be manipulated to understand disease pathways. For decades, targeted gene editing was not possible, limiting the ability of scientists to study individual genes. However, in the recent past, with the discovery of novel genetic engineering tools such as TALENs, ZFNs, and CRISPR-Cas9, researchers have overcome this challenge.
CRISPR-Cas9: Zebrafish as a Model System for Advancing Disease Research
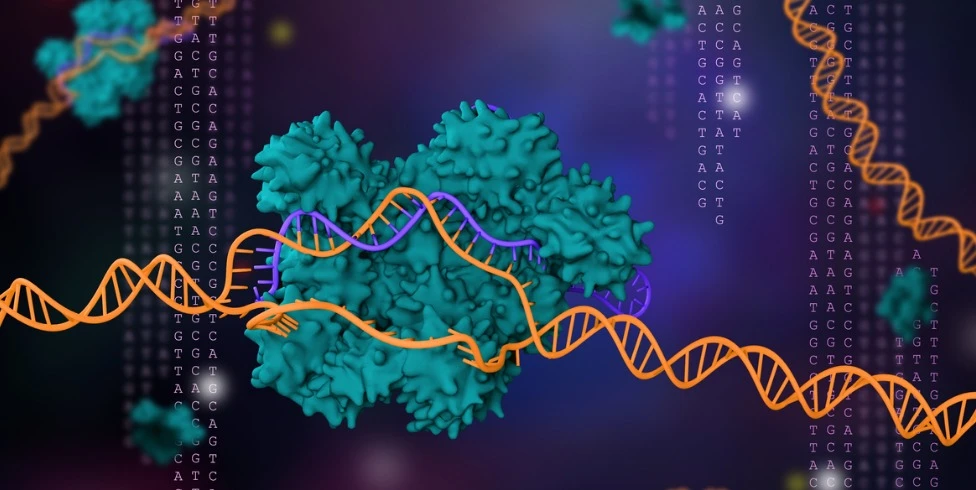
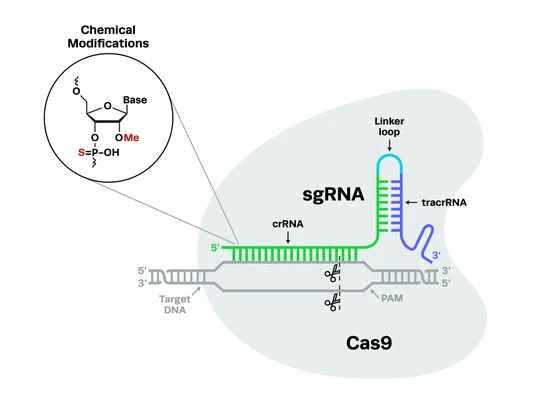
The study of model organisms is revolutionizing our understanding of human physiology and pathology of diseases. CRISPR-Cas9 technology has become a valuable tool for streamlining the generation of human disease models in zebrafish. Scientists have been coming up with new CRISPR zebrafish protocols to optimize gene editing. In this section, we cover how CRISPR studies in zebrafish are advancing scientific research.
CRISPR-Cas9 mediated Knock-in in Zebrafish
The mechanism of CRISPR-Cas9 involves introducing a double-stranded break in DNA at a targeted locus. Thereafter, cellular repair mechanism, homologous directed repair (HDR), is required for the integration of exogenous genes at the target locus. In a recent study, HDR-mediated knockin approach was used to generate a zebrafish model of amyotrophic lateral sclerosis (ALS) via the insertion of two SNPs.
Another team from Netherlands used CRISPR to generate four different knock-in lines carrying human cardiovascular-disorder-causing mutations (specifically related to Cantú syndrome) in their zebrafish orthologous genes. Through their study, they were able to demonstrate that the zebrafish knock-in lines displayed significantly enlarged ventricles with enhanced cardiac output, and distinct cerebral vasodilation, establishing a connection between the introduced mutations and Cantú syndrome.
CRISPR-mediated knock-in methods are slowly gaining popularity as it is sometimes important to introduce point mutations to replicate and understand human disease. Congenital heart defects (CHDs) are human disorders with complex genetics. A team based in Seattle is using CRISPR zebrafish models to understand if gene variants found in individuals with congenital heart defects contribute to the development of the disease.
CRISPR-Cas9 mediated Knockout in Zebrafish
Disruption of gene function in zebrafish can be commonly achieved via CRISPR knockouts. The approach that has demonstrated the highest editing efficiency is by microinjecting an in vitro complex of guide RNA and Cas9 protein into one-cell stage embryos. CRISPR-mediated knockout zebrafish models are extremely popular and have been used to model several diseases.
A recent study used CRISPR-mediated mutagenesis in zebrafish to study Fanconi Anemia (FA) pathway. They generated loss-of-function zebrafish knockout mutants for 17 FA genes. Their study revealed the role of these genes in growth, sexual development, and fertility.
Another team used zebrafish models to study the role of SHANK3 gene orthologs in autism spectrum disorder (ASD). Although previous studies have reported SHANK3 deficiency in ASD, the underlying molecular mechanism was not well understood. By using CRISPR to generate a shank3b loss-of-function mutation (shank3b −/− ) in zebrafish, the researchers were able to show that these mutant zebrafish displayed autism-like behavior.
The combination of zebrafish and CRISPR-Cas9 have the potential to become a powerful tool for the generation of disease models, validation of drug targets, and the discovery of therapeutics. However, there is a limit on the types of diseases that can be studied in zebrafish. Human genetic diseases that do not have a gene ortholog in zebrafish may require a different animal model. Also, zebrafish cannot be used to model human diseases that affect a body part that they do not possess. Stay tuned for future posts covering CRISPR research in other animal models in such cases.
We hope you found this post useful. If you are interested in learning more about CRISPR science, be sure to follow CRISPR in the news. You can also catch up with us on our blog, or follow us on Twitter or Facebook!