Contents
Human pluripotent stem cells are essential for preclinical and clinical research and applications. Over time, long-term cultured hPSCs can develop and accumulate recurrent genomic abnormalities and copy number variations that can be detrimental to basic research. Some copy number variations, such as the common 20q11.21 amplification, can lead to selective advantages and reduce differentiation capacities. Therefore, it is important to monitor and verify the genomic integrity of hPSC cultures as a key quality control measure.
What is Karyotyping and Why is it Important?
At its core, cytogenetics is a branch of genetics that studies the DNA structure within the cell’s nucleus. Karyotyping is a tool or technique that cytogeneticists use to study the structure of DNA in the nucleus. These approaches can vary from staining/imaging, sequencing, microarray, or polymerase chain reaction (PCR) based applications.
All these methods have their respective advantages and disadvantages that we will highlight in this blog.
Multiple Approaches to Karyotyping
Over the last several years, several new techniques and approaches have been used to perform karyotypic analysis. The turnaround times and resolutions of each approach are summarized in Table 1 below.
| Karyotyping Assay | Estimated TAT | Resolution |
G-banding (Staining) (Staining) |
3-4 wks | 5-10 Mb |
Whole Genome Sequencing |
6-8 wks | 1 bp |
Karyostat (Array Based Method) |
3-4 wks | 1-2 Mb |
Bionano (Optical genome imaging) |
6-8 wk | 0.2 kb |
KaryoLite BoBs |
1 wk* | 650Kb |
hPSC Genetic Analysis Kit (qPCR) |
1 wk* | 9 Targeted hot spots |
The iCS-digital PSC 24 probes kit (ddPCR) |
1 wk | 24 Targeted hot spots |
* External services are not available. TAT is based on internal resources and timelines.
Table 1. Turnaround times and resolutions for different karyotyping approaches
G-bandingG-banding or Giemsa banding is a DNA staining technique that is used to visually investigate the structure of condensed chromosomes within the nucleus of a cell. The Giemsa staining technique is named after the German chemist and bacteriologist Gustav Giemsa, who invented the technique in 1902 to detect malaria and Treponema pallidum in blood smears.
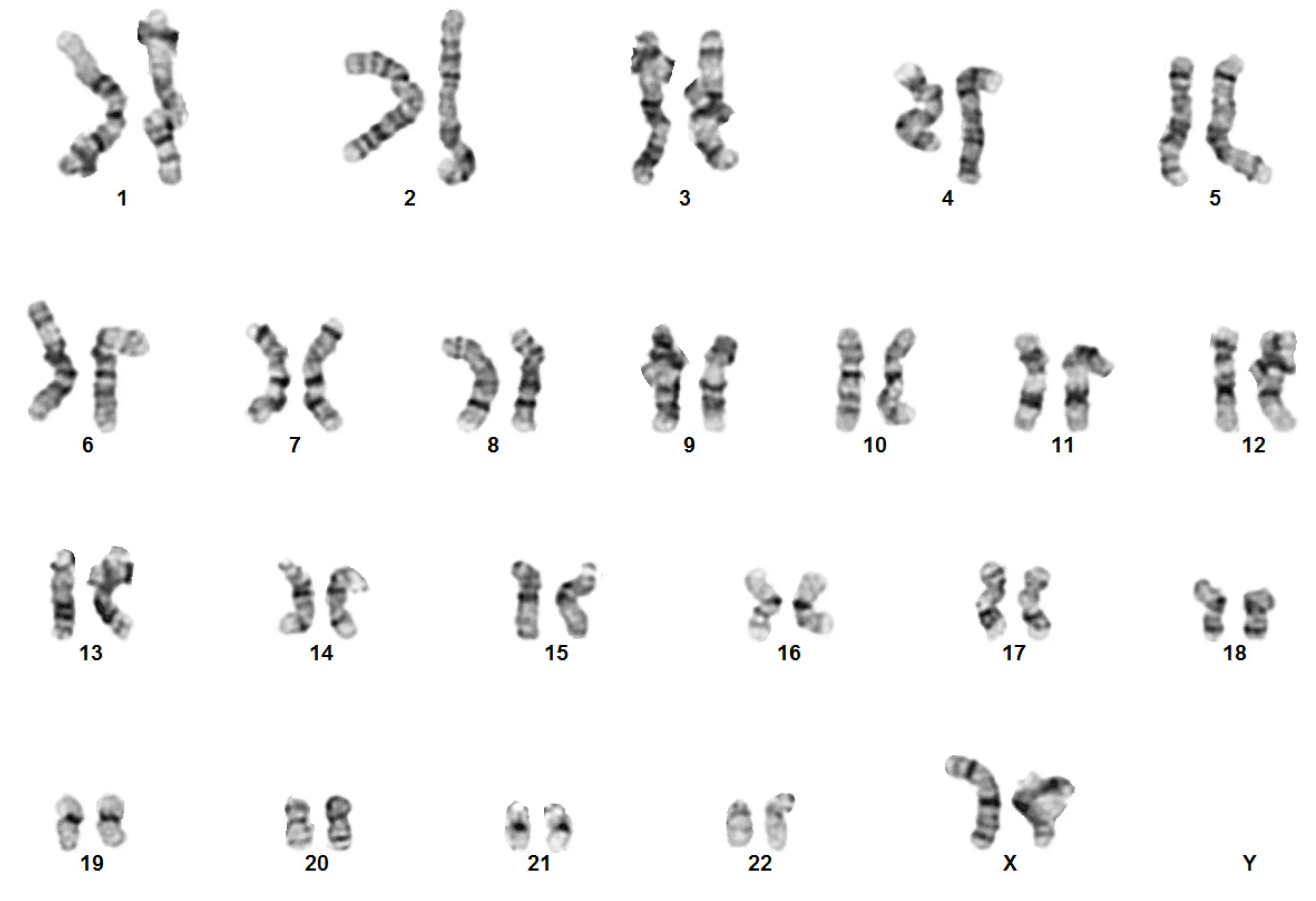
The basic principle of G-banding is that it is specific to the phosphate groups of DNA and attaches itself to regions of DNA where there are high amounts of adenine-thymine bonding, creating dark bands. Conversely, less condensed chromatin, which tends to be rich with guanine and cytosine, incorporates lesser Giemsa stain and appears as light bands. The result of G-banding creates a karyogram (chromosome map), which can be used to identify chromosomal aberrations such as translocations and rearrangements (Fig 1).
G-banding or Giemsa banding is a DNA staining technique that is used to visually investigate the structure of condensed chromosomes within the nucleus of a cell. The Giemsa staining technique is named after the German chemist and bacteriologist Gustav Giemsa, who invented the technique in 1902 to detect malaria and Treponema pallidum in blood smears.
The basic principle of G-banding is that it is specific to the phosphate groups of DNA and attaches itself to regions of DNA where there are high amounts of adenine-thymine bonding, creating dark bands. Conversely, less condensed chromatin, which tends to be rich with guanine and cytosine, incorporates lesser Giemsa stain and appears as light bands. The result of G-banding creates a karyogram (chromosome map), which can be used to identify chromosomal aberrations such as translocations and rearrangements (Fig 1).
Next-generation sequencingWith continued advancements in sequencing techniques and technology, another option for performing karyotypic analysis of your hPSCs is Next Generation Sequencing (NGS). NGS offers an alternative technology to karyotyping and due to its high resolution can accurately and precisely quantitate copy number variations.
With continued advancements in sequencing techniques and technology, another option for performing karyotypic analysis of your hPSCs is Next Generation Sequencing (NGS). NGS offers an alternative technology to karyotyping and due to its high resolution can accurately and precisely quantitate copy number variations.
What is next-generation sequencing?NGS is a high throughput process for determining DNA sequences. Briefly, platforms such as Illumina use sequence by synthesis (SBS) chemistry and DNA polymerase to catalyze fluorescently labeled nucleotides into a DNA template strand during sequential cycles of DNA synthesis. During each cycle, nucleotides are identified by their specific fluorophore labels. This process is highly scalable and can be targeted to hotspot regions of DNA known to be problematic in the context of karyotyping.
NGS is a high throughput process for determining DNA sequences. Briefly, platforms such as Illumina use sequence by synthesis (SBS) chemistry and DNA polymerase to catalyze fluorescently labeled nucleotides into a DNA template strand during sequential cycles of DNA synthesis. During each cycle, nucleotides are identified by their specific fluorophore labels. This process is highly scalable and can be targeted to hotspot regions of DNA known to be problematic in the context of karyotyping.
Basic karyotyping NGS workflow:
- Library preparation: The sequencing library is generated by creating short DNA or cDNA fragments with 5’ and 3’ adapters ligated.
- Cluster amplification: The library is loaded onto a flow cell, and cDNA fragments are hybridized to the flow cell surface via bridge amplification. Each fragment acts as a seed to generate identical clonal clusters containing thousands of identical fragments.
- Sequencing: Using a reversible terminator-based method, SBS technology detects single bases as they are incorporated into the DNA template strands.
- Alignment and data analysis: Sequence reads are exported to an output file and aligned against a reference genome by alignment software (Fig 2).
- Library preparation: The sequencing library is generated by creating short DNA or cDNA fragments with 5’ and 3’ adapters ligated.
- Cluster amplification: The library is loaded onto a flow cell, and cDNA fragments are hybridized to the flow cell surface via bridge amplification. Each fragment acts as a seed to generate identical clonal clusters containing thousands of identical fragments.
- Sequencing: Using a reversible terminator-based method, SBS technology detects single bases as they are incorporated into the DNA template strands.
- Alignment and data analysis: Sequence reads are exported to an output file and aligned against a reference genome by alignment software (Fig 2).

While the use of NGS for karyotyping can be extremely powerful, it is often cost-prohibitive compared to standard laboratory practices as it involves cell culture. Further, another limitation of NGS is that small fragmentations in WGS prevents revealing any large structural changes in the genome that can be detected with other methodologies such as G-banding.
Array-based karyotyping: KaryoStat™ and KaryoStatS+™Array-based karyotyping technology is a powerful tool for assessing chromosomal copy number changes that provide information not previously obtainable by conventional karyotyping techniques such as G-banding. This is the method currently used in Synthego analysis.
Array-based karyotyping technology is a powerful tool for assessing chromosomal copy number changes that provide information not previously obtainable by conventional karyotyping techniques such as G-banding. This is the method currently used in Synthego analysis.
What is array-based karyotyping?Array-based karyotyping is an alternative to G-band karyotyping that offers the same whole-genome coverage for the accurate detection of chromosomal abnormalities. The development of array-based technology stemmed from the fact that traditional methodologies such as G-banding and FISH techniques were limited in the potential resolution by the microscopes necessary for interpretation of the results they provided. However, array-based karyotyping is only able to detect unbalanced chromosomal abnormalities, and it cannot detect balanced abnormalities such as reciprocal translocations, inversions, or ring chromosomes, as these abnormalities do not affect copy number, which is what is detected by this technology.
Array-based karyotyping is an alternative to G-band karyotyping that offers the same whole-genome coverage for the accurate detection of chromosomal abnormalities. The development of array-based technology stemmed from the fact that traditional methodologies such as G-banding and FISH techniques were limited in the potential resolution by the microscopes necessary for interpretation of the results they provided. However, array-based karyotyping is only able to detect unbalanced chromosomal abnormalities, and it cannot detect balanced abnormalities such as reciprocal translocations, inversions, or ring chromosomes, as these abnormalities do not affect copy number, which is what is detected by this technology.
Array-based workflow
- DNA labeling: DNA is extracted from the sample(s) and labeled with a fluorescent dye.
- Hybridization: Mix and hybridize to an array printed with thousands of oligonucleotide probes and wash.
- Fluorescence visualization and imaging: Detect signals with a fluorescence scanner and compute and report gains and losses by comparing them to a reference genome.
- DNA labeling: DNA is extracted from the sample(s) and labeled with a fluorescent dye.
- Hybridization: Mix and hybridize to an array printed with thousands of oligonucleotide probes and wash.
- Fluorescence visualization and imaging: Detect signals with a fluorescence scanner and compute and report gains and losses by comparing them to a reference genome.
Limitations of array-based technologiesThere are many positives to using array-based methodologies for karyotyping. For example, these assays often provide whole-genome coverage. However, because of this great breadth in coverage, there is a sacrifice in resolution. Notably, chromosomal gains and deletions are often limited by detection limits of greater than 1Mb. While this is still better than traditional G-banding which has detection limits set at 5Mb, it is not as accurate as NGS and ddPCR methods which have lower detection limits.
There are many positives to using array-based methodologies for karyotyping. For example, these assays often provide whole-genome coverage. However, because of this great breadth in coverage, there is a sacrifice in resolution. Notably, chromosomal gains and deletions are often limited by detection limits of greater than 1Mb. While this is still better than traditional G-banding which has detection limits set at 5Mb, it is not as accurate as NGS and ddPCR methods which have lower detection limits.
Digital droplet polymerase chain reactionsA newer development for investigating chromosomal abnormalities emerged with the development of digital droplet PCR (ddPCR) for karyotypic analysis. The ddPCR technology uses similar reagents and workflows to that of traditional TaqMan probe-based assays, however, it incorporates massive sample partitioning, which is a key aspect of the ddPCR technique.
A newer development for investigating chromosomal abnormalities emerged with the development of digital droplet PCR (ddPCR) for karyotypic analysis. The ddPCR technology uses similar reagents and workflows to that of traditional TaqMan probe-based assays, however, it incorporates massive sample partitioning, which is a key aspect of the ddPCR technique.
What is digital droplet PCR?Digital droplet PCR is a refinement of the conventional PCR technique. However, the key difference between the two techniques lies within the methods for quantifying nucleic acids. While ddPCR can be more precise with its measurements, it can be more error-prone to those who are inexperienced in using this technique.
Like NGS, in karyotyping with ddPCR, probes can be designed to hotspot regions in the DNA that are known to be problematic and where genomic abnormalities commonly occur. One such example of this approach is the iCS-digital™ PSC 24 probe kit from Stem Genomics.
Digital droplet PCR is a refinement of the conventional PCR technique. However, the key difference between the two techniques lies within the methods for quantifying nucleic acids. While ddPCR can be more precise with its measurements, it can be more error-prone to those who are inexperienced in using this technique.
Like NGS, in karyotyping with ddPCR, probes can be designed to hotspot regions in the DNA that are known to be problematic and where genomic abnormalities commonly occur. One such example of this approach is the iCS-digital™ PSC 24 probe kit from Stem Genomics.
The ddPCR workflow
- Sample preparation: DNA from sample cells is combined with primers, probes, and ddPCR supermix
- Droplet generation: Samples are loaded onto a droplet generating machine in which ~20,000 monodispersed PCR-ready droplets are created.
- PCR amplification of droplets: Droplets are transferred to a 96-well plate for PCR amplification in any compatible thermal cycler.
- Droplet reading: Droplets are placed into a droplet reader in which each droplet is analyzed individually using a two-color detection system. This allows for multiplexed analysis for different targets from the same sample.
- Analysis and visualization of results: Positive droplets containing at least one copy of the target exhibit increased fluorescence over negative droplets. The fraction of positive droplets is then fitted to a Poisson distribution to determine the absolute initial copy number of the target DNA molecule in the input reaction mixture in units of copies/µl. Manufacture-specific ddPCR software allows you to visualize the data in a variety of ways to determine the concentration in copies/µl.
- Sample preparation: DNA from sample cells is combined with primers, probes, and ddPCR supermix
- Droplet generation: Samples are loaded onto a droplet generating machine in which ~20,000 monodispersed PCR-ready droplets are created.
- PCR amplification of droplets: Droplets are transferred to a 96-well plate for PCR amplification in any compatible thermal cycler.
- Droplet reading: Droplets are placed into a droplet reader in which each droplet is analyzed individually using a two-color detection system. This allows for multiplexed analysis for different targets from the same sample.
- Analysis and visualization of results: Positive droplets containing at least one copy of the target exhibit increased fluorescence over negative droplets. The fraction of positive droplets is then fitted to a Poisson distribution to determine the absolute initial copy number of the target DNA molecule in the input reaction mixture in units of copies/µl. Manufacture-specific ddPCR software allows you to visualize the data in a variety of ways to determine the concentration in copies/µl.
Limitations with ddPCR techniqueThere are certain limitations to ddPCR-based technology for karyotyping. In general, while the depth (resolution) of coverage achieved with ddPCR is greater than that of array-based technologies, you do not get the breadth of whole-genome coverage as seen with other technologies. That said, using commercially available kits, such as those available from Stem Genomics which target 24 of the most common hotspots, can provide genomics stability information for regions that are associated with ~90% of the genomic abnormalities.
There are certain limitations to ddPCR-based technology for karyotyping. In general, while the depth (resolution) of coverage achieved with ddPCR is greater than that of array-based technologies, you do not get the breadth of whole-genome coverage as seen with other technologies. That said, using commercially available kits, such as those available from Stem Genomics which target 24 of the most common hotspots, can provide genomics stability information for regions that are associated with ~90% of the genomic abnormalities.
Use Cases With Improved Resolution Leveraging ddPCR Analysis
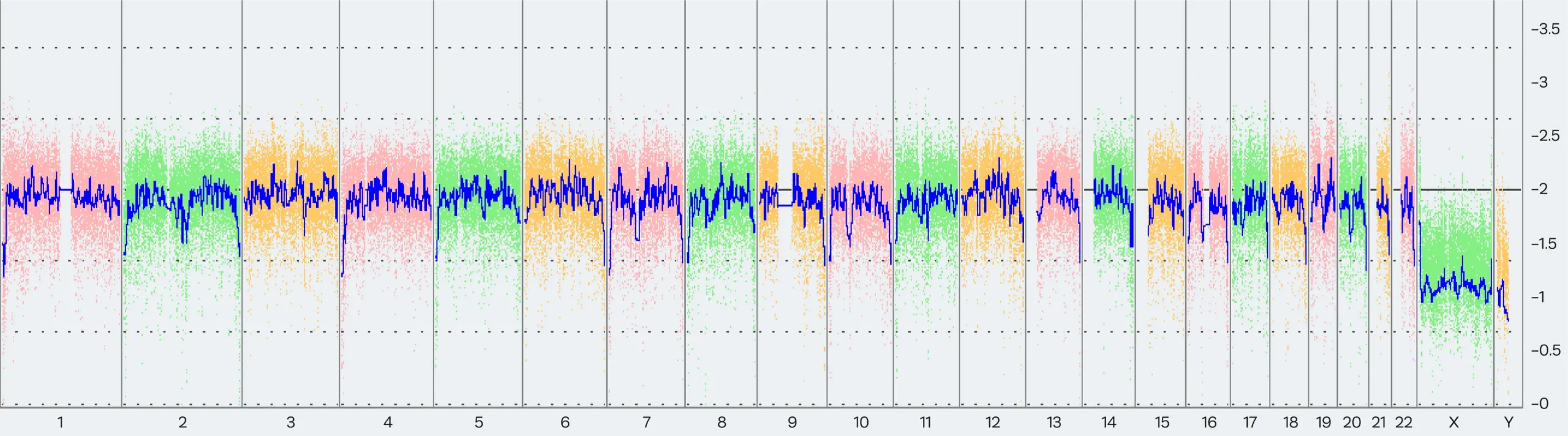
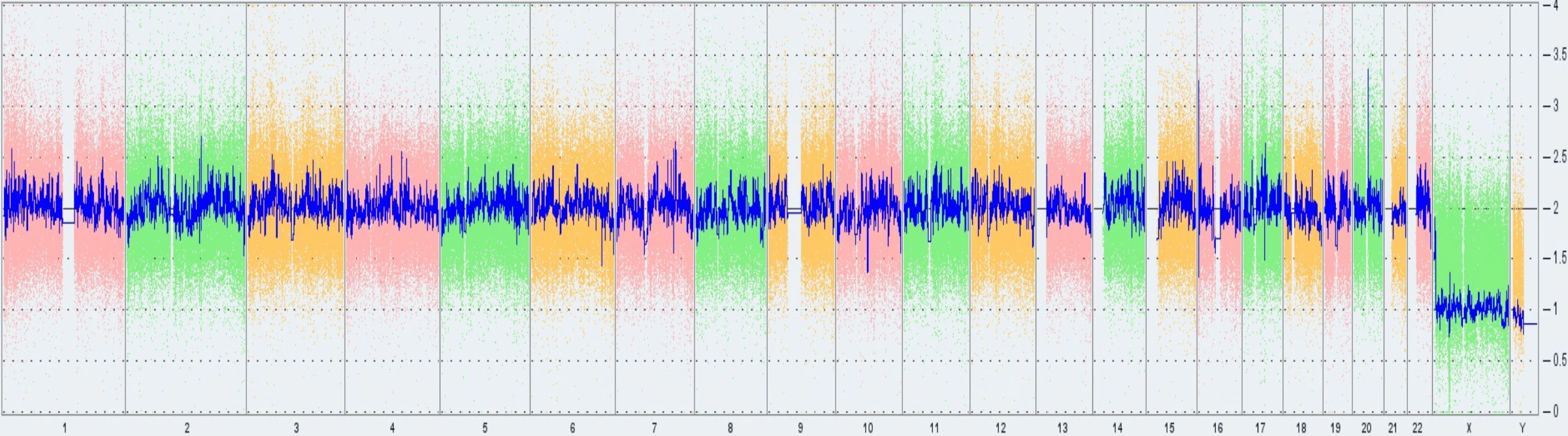
As highlighted above, each of the current karyotyping methodologies has its respective benefits and limitations. In this case study, we will highlight the key differences between two methods. As standard for Synthego, we use Thermo Fisher’s karyotyping services to perform routine karyotyping for our Synthego-supplied PGP-1 cell lines (Fig 5).
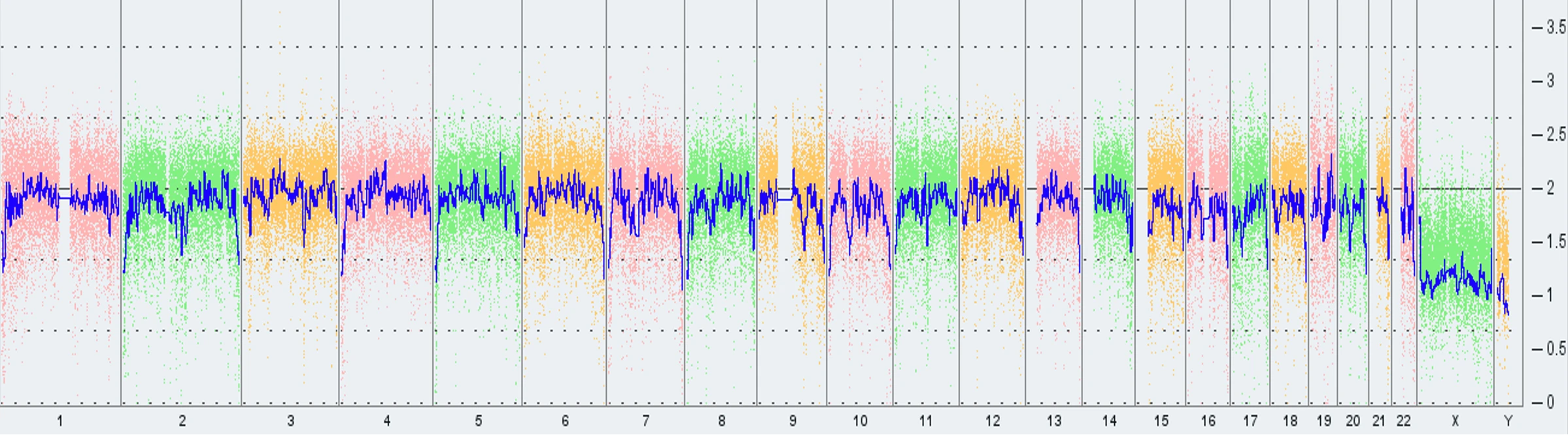
However, as of February 2022, Thermo Fisher updated their array-based karyotyping service platform from KaryoStat™ to KaryoStat+™. With this update, the resolution for detecting chromosomal gains improved from > 2 Mb to > 1 Mb.
As a result, Synthego detected the presence of the common 20q11.21 amplification, a sub-chromosomal abnormality on chromosome 20, in some PGP1 edited clones and in our parental cell bank.
What specifically is the 20q11.21 amplification abnormality?
- Gain of 20q11.21 copy-number variant (CNV) is detected in more than 20% of worldwide cultured hPSCs. (Halliwell et al., Baker et al., Avery et al.)
- The most common genomic abnormality that represents 22.9% of the recurrent structural variants identified in hPSCs. (Assou et al., 2020)
- Cells with the 20q11.21 gain have a selective advantage and can completely overtake a culture within a few passages. (Assou et al., 2018)
- Most 20q11.21 CNVs fall below the size detection limit of conventional G-banding and microarray-based detection methods.
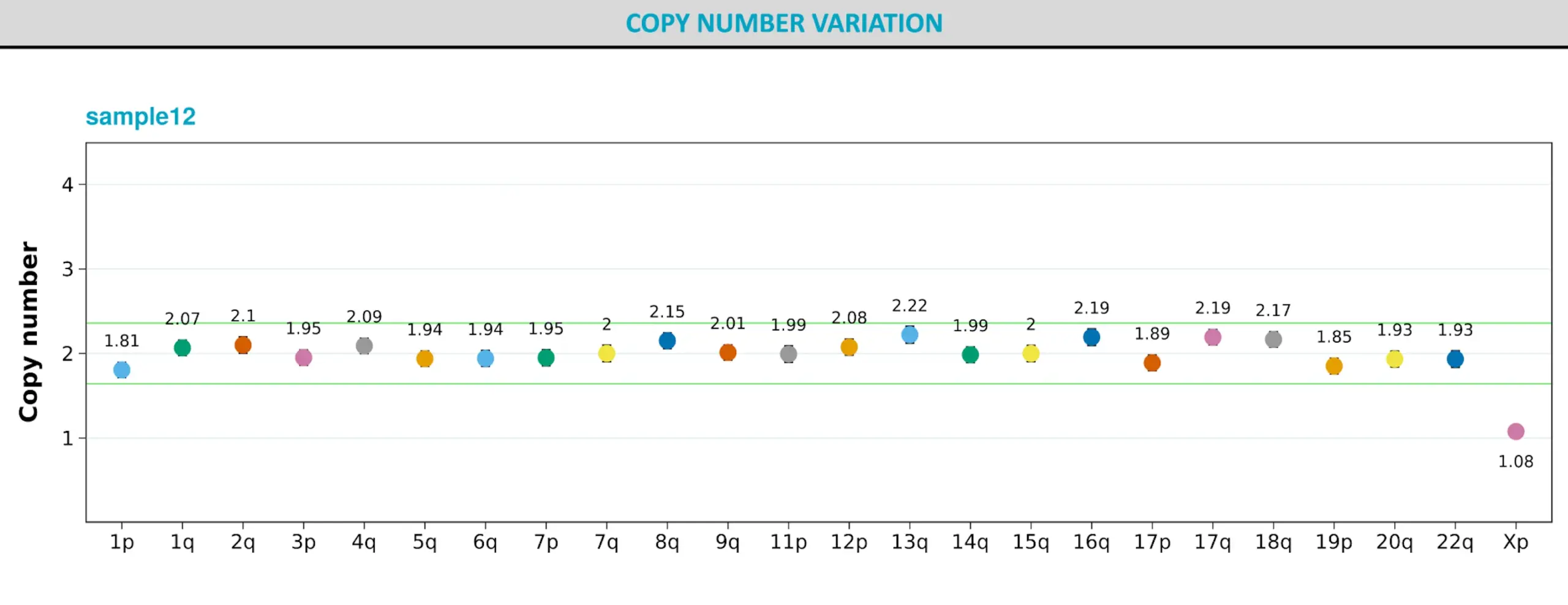
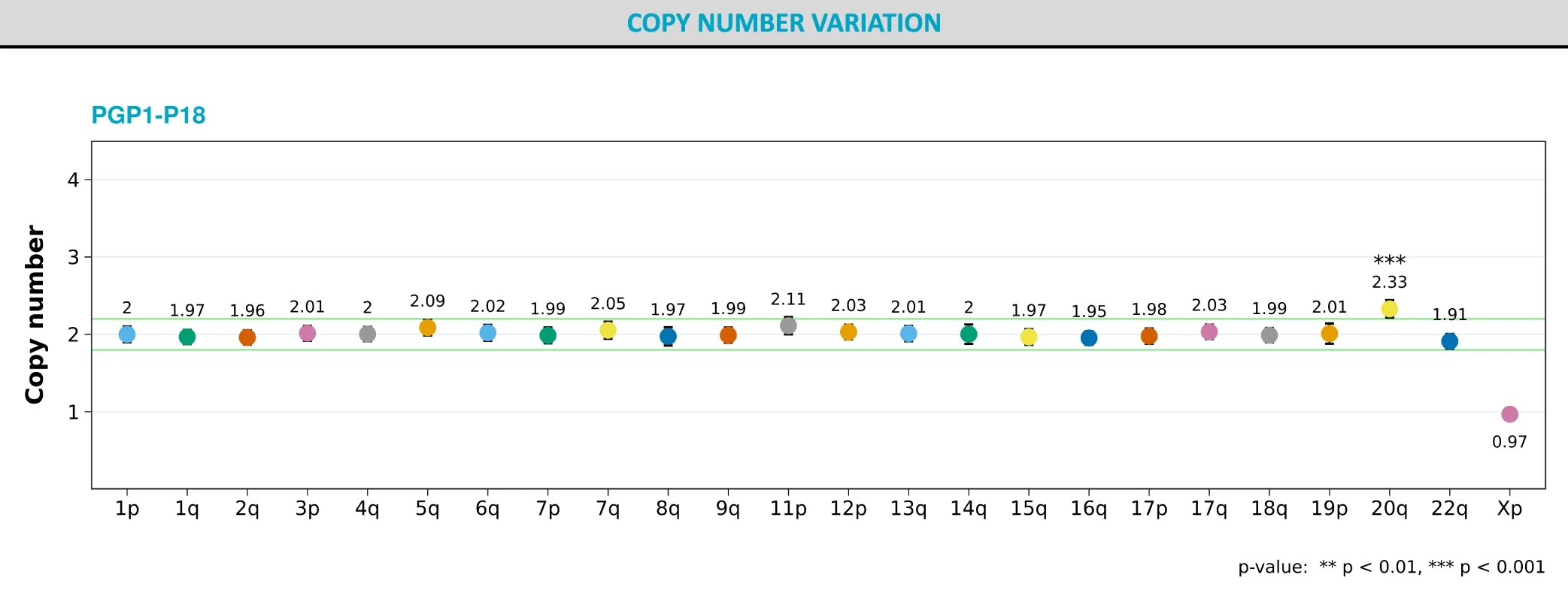
Further, to confirm these results, we performed ddPCR karyotyping on our parental cell lines using Stem Genomics iCS-digital™ PSC 24 probe kit and confirmed the results of the new KaryoStat+ test (Fig 6).
- Gain of 20q11.21 copy-number variant (CNV) is detected in more than 20% of worldwide cultured hPSCs. (Halliwell et al., Baker et al., Avery et al.)
- The most common genomic abnormality that represents 22.9% of the recurrent structural variants identified in hPSCs. (Assou et al., 2020)
- Cells with the 20q11.21 gain have a selective advantage and can completely overtake a culture within a few passages. (Assou et al., 2018)
- Most 20q11.21 CNVs fall below the size detection limit of conventional G-banding and microarray-based detection methods.
Further, to confirm these results, we performed ddPCR karyotyping on our parental cell lines using Stem Genomics iCS-digital™ PSC 24 probe kit and confirmed the results of the new KaryoStat+ test (Fig 6).
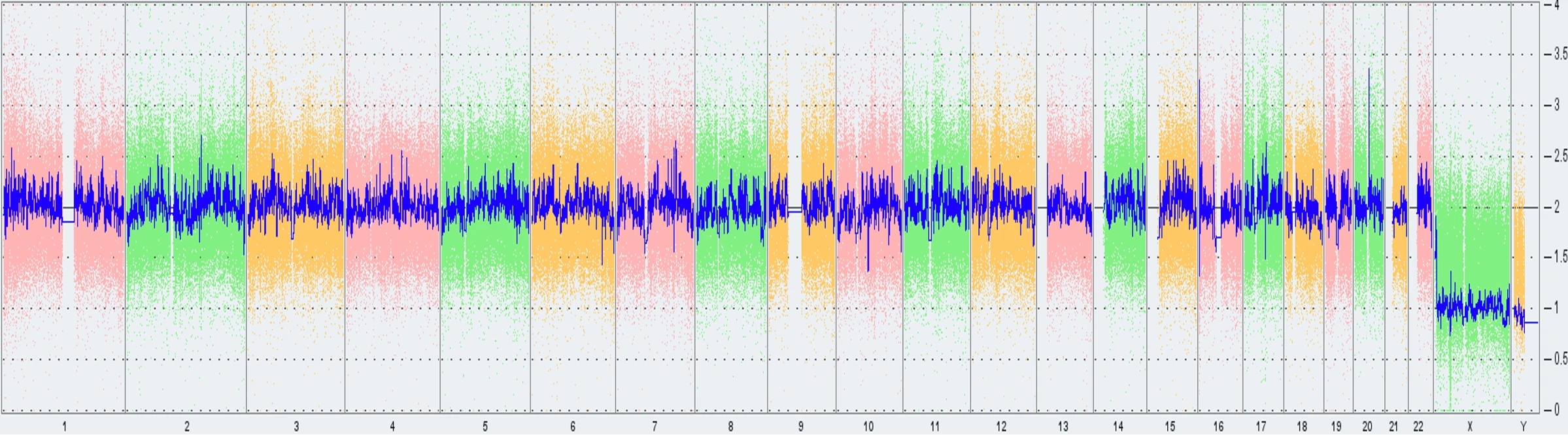
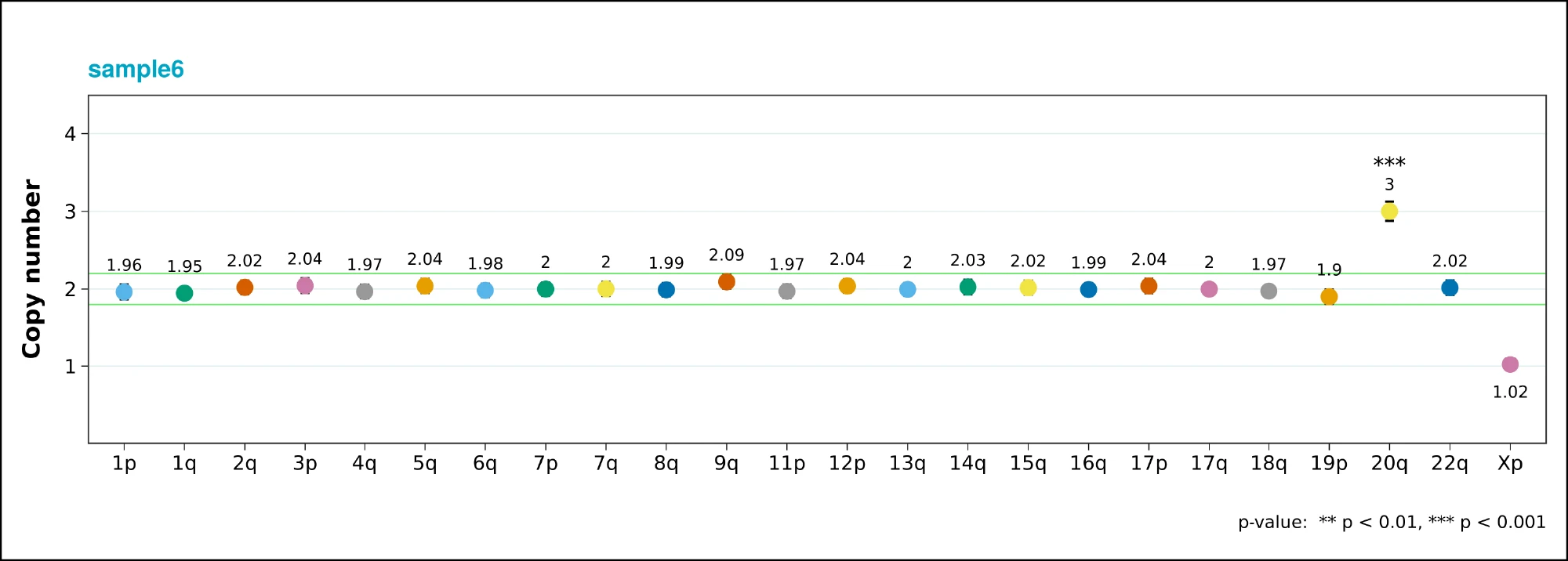
In an attempt to proactively investigate the root cause of this abnormality, Synthego sourced another low-passage vial of PGP1 and had it tested for the same abnormality. Interestingly, Thermo Fisher’s KaryoStat+ analysis produced a negative result. However, when ddPCR analysis was performed on the same sample, the results came back as abnormal for the 20q abnormality. We hypothesize that the abnormality is present at low levels in the parental population and is therefore below the detection limit of the array-based technology. However, when targeted specifically with the ddPCR based methodology, this abnormality becomes apparent (Fig 7).
The Future of Karyotyping
With continued advancement in karyotyping technology, similar to what have seen over the past decade, we suspect that further chromosomal abnormality will be discovered in many more hPSCs currently being used in research labs across the globe.
High precision in karyotyping methods will provide deeper insights and thus help scrutinize and maintain the hPSC genomic integrity which is crucial to research and clinic.