The introduction of CRISPR, a precise genome editing tool, has been a game changer for the scientific community. One of the most exciting applications of CRISPR is in cell and gene therapy. In fact, CRISPR-mediated clinical trials for a few diseases—sickle cell anemia, beta thalassemia, and cancer, to name a few—are already underway.
As these therapies largely rely on tedious and expensive viral delivery of CRISPR components, research on alternative, scalable non-viral delivery techniques is pertinent. Indee Labs, a biotech in Berkeley led by CEO Ryan Pawell, is doing just that. The company focuses on a novel, patented approach for the delivery of CRISPR components into cells: microfluidic vortex shedding.
"The mission of our company is to apply our technology to replace viral manufacturing in the development workflows of T-cell immunotherapies to dramatically decrease the costs associated with their manufacturing and make them available to more patients"
Justin Jarrell, Ph.D., Director of Research at Indee Labs.
In a step closer to this goal, the Indee Labs team recently demonstrated successful CRISPR editing of TRAC-1 in T cells with their optimized microfluidic vortex shedding platform.
Read more about the summary of their work, published in BioRxiv, in this blog post.


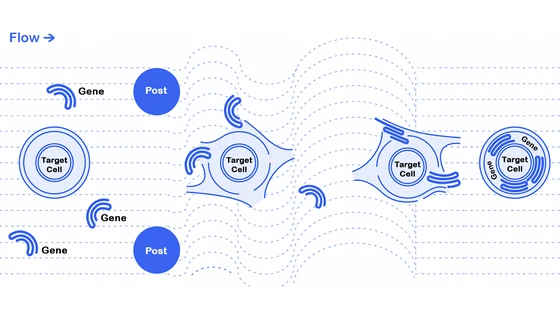
Microfluidic Vortex Shedding: A Brief Intro
Vortex shedding is a phenomenon resulting from the flow of a fluid past an obstacle, creating alternating low-pressure in downstream regions. Examples of vortex shedding on a macro scale include river water flowing over rocks or clouds flowing over low mountains.
Researchers can mimic these dynamics on a scale of 1/10 to 1/100 the thickness of the human hair—the size of cells—using microfluidic devices. These are tiny channels used to manipulate fluids; in this particular case, Dr. Jarrell and team induced vortex shedding past an obstruction, called a post, in their channels.
The concept of using microfluidic vortex shedding (µVS) as a delivery system in cells was born years ago when Ryan Pawell, CEO of Indee Labs and senior author of this publication, was working on a cell separation technology. The process involved pushing cells through tiny little post arrays to separate big cells from the small cells. In one of his experiments, Pawell realized that the hydrodynamic conditions created by µVS made the cell membrane transiently permeable, opening a new avenue for the delivery of small molecules into cells.

CRISPR Editing of T cells Using Microfluidic Devices
In a previous study, the Indee Labs team had shown that mRNA could be delivered into human primary T cells using their microfluidics platform. In their recent publication, the researchers dug deeper into the optimization parameters contributing to vortex shedding. The team characterized µVS samples across more than 150 parameters and used simulations to identify the 11 most predictive parameters that could be tweaked to increase vortex shedding duration, intensity, length of time, etc.
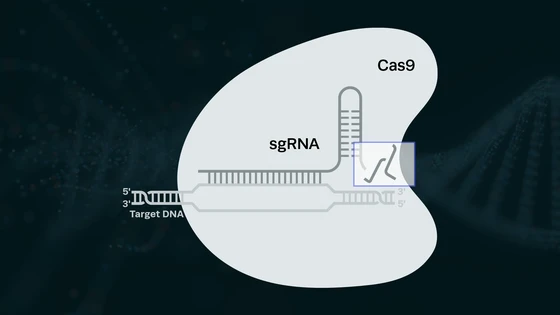
On testing µVS designs with a splitter plate between post columns to attenuate downstream vortices, Dr. Jarrell noted that shorter splitter plates correlated with increased delivery efficiency. This was quantified by means of eGFP mRNA delivered into activated primary human T cells. With the proof of principle established, the team then wanted to test the efficacy of actual CRISPR editing. They chose Synthego’s synthetic sgRNA targeting the T cell receptor alpha locus (TRAC-1); Synthego’s high-quality guides are known to be highly effective for editing primary T cells.
On delivering the sgRNA and Cas9 protein in the ribonucleoprotein (RNP) format, the researchers observed >35% editing efficiency, indicating successful knockout of the TRAC-1. Importantly, at this time point, >80% of T cells were viable, confirming the minimal negative impact of this delivery method.
Scalable Microfluidic Devices Are the Future of CRISPR Therapeutics
Now that the proof of principle is established, the team hopes to develop more such devices in the future to expand applications to cellular immunotherapies, as the ultimate goal is to get these devices into the manufacturing workflows.
The microfluidic vortex shedding technology offers the best of both worlds of viral delivery and electroporation—the current gold standards for delivery—according to Dr. Jarrell. The gentle nature of their platform offers the ease of electroporation without the associated negative effects, but researchers can still scale up as they would with viral manufacturing without worrying about the associated costs and safety issues.
"We can make thousands [of devices] per day with existing manufacturing workflow. Current 5 x 10 mm devices process tens of millions of cells in seconds and we are developing one that can process over a billion cells in a similar timeframe and with a similar footprint. So, from a comprehensive perspective, we have the ideal technology for developing these T cell immunotherapies at scale."
Ryan Pawell, CEO, Indee Labs.