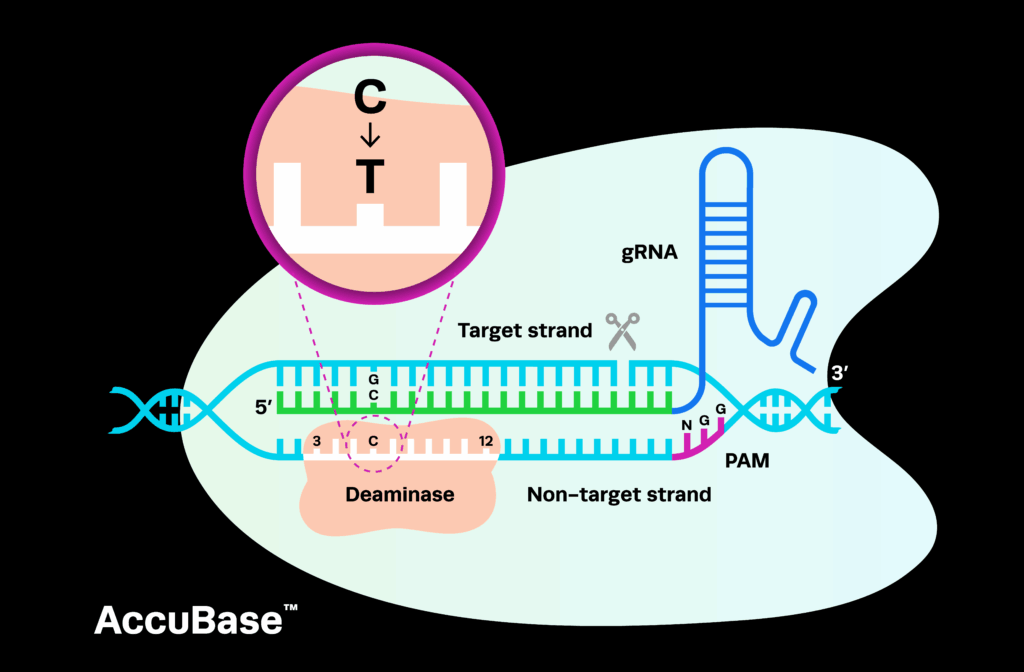
The precise CRISPR genome editing tool has shown promising potential in developing gene therapies against monogenic diseases. Researchers are particularly invested in developing therapies for blood disorders, such as sickle cell anemia and beta thalassemia, due to the technological enablement of ex vivo cellular therapies.
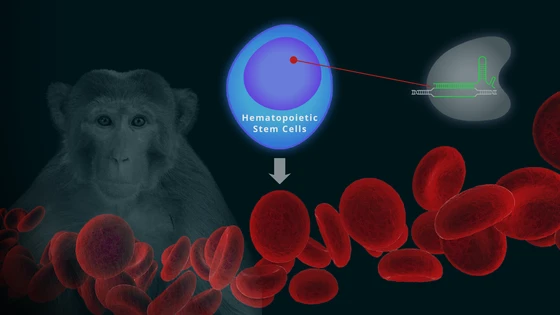
In the latest stride in this direction, researchers at the Fred Hutchinson Cancer Research Center have reported a new CRISPR-based gene therapy approach for editing hematopoietic stem cells (1). The team validated their method by demonstrating long term replenishment of fetal hemoglobin levels in rhesus monkeys.
“The approach we are describing is completely translatable to the clinic,” Dr. Olivier Humbert, a researcher at the Fred Hutchinson Cancer Research Center and first author of the paper. Read the summary of his work below.
A Gene Therapy Approach to Treat Blood Disorders
Beta-hemoglobinopathies, such as sickle cell anemia and beta thalassemia, are caused by mutations in the beta-globin gene. An upcoming gene therapy strategy is to edit hematopoietic stem cells—precursors that produce blood cells—as a one-time treatment for patients.
One way of restoring hemoglobin in cells is by activation of fetal hemoglobin (normally repressed in adulthood) that can functionally substitute adult hemoglobin. The recent CRISPR-based CTX001 gene therapy trials to treat beta thalassemia use this strategy by directly inactivating the repressor of fetal hemoglobin, BCL11A.
Dr. Humbert and his colleagues developed an alternative strategy to increase fetal hemoglobin levels in the blood; they targeted the sequence in the promoter region that the repressor BCL11A binds. The team chose rhesus monkeys for their experiments, as their genome closely resembles the human genome.
The team first tested their approach in vitro. Blood stem cells were isolated from healthy monkeys, and edited using chemically modified sgRNA from Synthego, and Dr. Humbert notes that "the editing efficiency went up pretty dramatically" as compared to in vitro-transcribed RNA that they had used before. Once the process was validated in vitro, evidenced by higher fetal hemoglobin levels in differentiated erythroids, the next step was to test the long term success of this strategy by engrafting the edited cells back into the monkeys.
CRISPR & Monkey Business: Engrafting Edited Stem Cells in Animals
The researchers confirmed fetal hemoglobin reactivation by examining the peripheral blood of the monkeys and noted up to 18% fetal hemoglobin producing cells on monitoring them for more than a year. The team also reported normal counts of blood cells of all lineages and the absence of off-target effects in the animals. These preliminary results indicate that this strategy could be a promising approach for gene therapy in humans in the future.
Another novel component of Dr. Humbert’s work was the successful engraftment of their “redefined stem cells.” The current gold standard for defining blood stem cells is by selecting for expression of the CD34+ receptor.
"We know that this is not a perfect model because among CD34+ cells there are also precursor cells and more differentiated cells "
said Dr. Humbert.
As the cost of ex vivo CRISPR therapies is very high, any approaches that could reduce the required reagents would make the process cheaper.
The team had previously found that using three cell markers, CD34+CD90+CD45RA−, they could narrow down the definition of blood stem cells (2). Therefore, they engrafted these edited triple marker cells in monkeys, and found that they needed 10-fold fewer cells, as compared to the CD34+ approach, to achieve comparable results.
In this preclinical work, the researchers have presented a cheaper and competent approach to gene therapy for blood disorders. The researchers will keep monitoring the monkeys for more years to confirm the efficacy and safety of this process. In the future, moving to human trials would be the ideal next step with the help of industry partners, said Dr. Humbert.
Publications
1) Humbert, Olivier, et al. "Therapeutically relevant engraftment of a CRISPR-Cas9–edited HSC-enriched population with HbF reactivation in nonhuman primates." Science translational medicine 11.503 (2019): eaaw3768.
2) Radtke, Stefan, et al. "A distinct hematopoietic stem cell population for rapid multilineage engraftment in nonhuman primates." Science translational medicine 9.414 (2017): eaan1145.