About 7 years ago, a paper explaining the biochemical mechanism of gene editing tool called CRISPR made waves in the field of genome engineering. This tool provided a way to precisely edit genomes with relative ease as compared to previous methods, and thus showed great potential to advance medicine, agriculture, and more. While CRISPR has certainly lived up to its expectations, there are certain limitations that stand in the way for achieving its full potential.
For instance, CRISPR-mediated DNA cleavage is just half the story; currently, CRISPR genome editing relies on the repair mechanisms of cells. Researchers have been exploring ways in which they could maintain the precision of CRISPR but reduce the unreliable aspect of cellular repair.
Samuel Sternberg, Assistant Professor at Columbia University, and his team have now found an alternative way for inserting desired DNA sequences into precise genomic locations without a double-stranded break. Read on to learn about their work on INTEGRATE technology, which combines transposons and CRISPR, published in Nature recently.
CRISPR-Cas9 System Relies on Cellular Repair Pathways
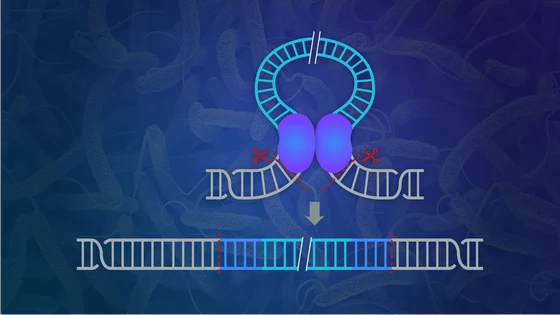
CRISPR became a popular tool primarily due to its simplicity. Typically, the guide RNA targets specific DNA sequence and a Cas nuclease cleaves at the precise location by generating a double-standard break. The cellular repair pathways do the rest. For example, the cell may recruit the non-homologous end joining (NHEJ) pathway for a quick repair. An error in the repair pathway, wherein a few nucleotides are inserted or deleted (indels), is the basis of disrupting gene function, i.e. knockouts.
Obtaining knock-in—insertion or substitution of gene fragments at precise locations—is even more challenging. The cell employs the homology-directed repair (HDR) pathway, which requires a donor template to accurately copying missing information during strand repair. As HDR occurs only in specific cell division phases and is not very reliable, it is highly challenging to obtain CRISPR knock-ins.
Jumping Genes Integrate into the Genome Without HDR
Transposons are mobile genetic elements that can “jump” positions in the genome. In fact, transposons have also been shown to be a part of CRISPR evolution with a role in spacer acquisition and RNA-guided targeting.
In 2017, Sternberg, who had been following the decades of seminal work on transposons and CRISPR, came across an interesting paper by Joseph Peters from Cornell University. Peters and team had performed a phylogenetic analysis of several bacterial systems and found that T7-like transposons associate with Type 1 CRISPR system.
“Transposases generally fall into two categories, either they integrate randomly or they integrate with very inflexible specificity,” explained Sternberg. But this T7-like transposase system lacked a key gene involved in DNA targeting, and the transposon-encoded CRISPR-Cas systems lacked a key gene involved in DNA degradation. This led to the hypothesis that these transposon-encoded CRISPR–Cas systems must leverage DNA targeting via guide RNAs to aid transposon insertion at different sites in the genome.
“These links between nuclease deficient CRISPR Cas systems that co-occur with transposable elements looked very interesting to us and immediately suggested a possibility for using those systems as new tools for programmable insertion,” said Sternberg. He and his team embarked upon the mission of making this possibility into reality.
Developing a Programmable Transposon-CRISPR System
Sternberg and team zeroed in on Vibrio cholerae transposase system for their first study after extensive bioinformatics analyses and screening of different transposon systems. They introduced the transposable elements such as donor DNA and required proteins from V. cholerae into E.coli via plasmids. The team demonstrated that they could use programmable donor sequences and achieve integrations of up to 10 kb DNA guided by CRISPR in bacteria with high fidelity.
This is the first time that transposases have been programmed for targeted DNA insertion in conjunction with CRISPR. Additionally, the team also performed extensive experiments to understand the underlying mechanism of this combined system and found that a key player- a transposition protein, TniQ.
"This link between the particular CRISPR complex called Cascade and TniQ, showing that they functionally interact with each other, begins to tell us exactly how these two machineries are evolving,” said Sternberg.
Will the Transposase-CRISPR System Jump into Mammalian Cells?
Sternberg’s team is not the only one working on this system. Around the same time, Feng Zhang’s lab also published a similar study on transposable elements combined with CRISPR in bacteria in Science.
While the proof of principle has been shown in bacteria in both studies, the obvious question is “Can this be translated to mammalian cells?” Both teams believe that it can.
According to Sternberg, while researchers will need to figure our delivery and expression questions when moving from bacterial to eukaryotic system, these are not real obstacles. Given the progress in CRISPR in the past 7 years, he believes this problem should be easily solvable. Sternberg’s team is also working on improving other aspects of the system.
“We are excited to be discovering as many new things as we can and add it to the CRISPR toolbox to give the community all the components they might want to start tinkering around and making these systems even more effective,” said Sternberg.
Original PublicationKlompe, Sanne E., et al. "Transposon-encoded CRISPR–Cas systems direct RNA-guided DNA integration." Nature (2019): 1.
Klompe, Sanne E., et al. "Transposon-encoded CRISPR–Cas systems direct RNA-guided DNA integration." Nature (2019): 1.