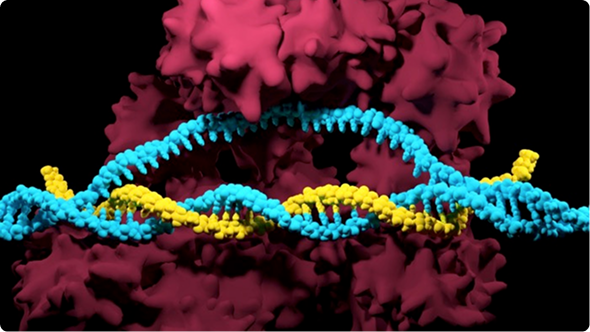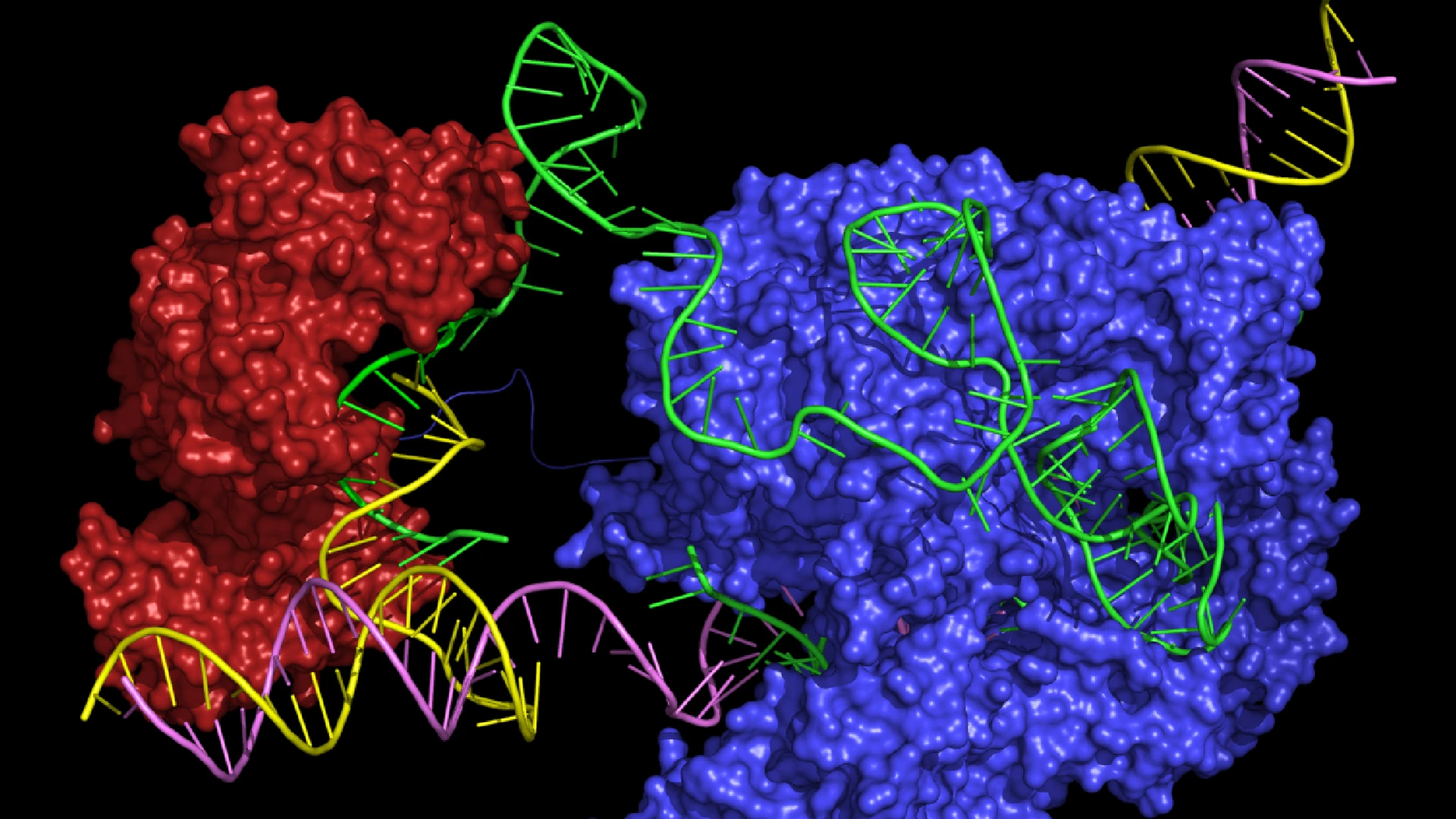
Image courtesy of Peyton Randolph and Andrew Anzalone. Note this image is a model of the prime editing complex, not the actual structure.
CRISPR has revolutionized gene editing by enabling a level of precision previously unattainable. Yet, the technology is not without limitations, making the transition from lab research to clinical applications for gene and cell therapies come with some challenges. Enter base editing and prime editing, two groundbreaking precision gene editing techniques that build on CRISPR to address these hurdles. Base editing allows for precise modifications of single nucleotides without introducing double-strand breaks, while prime editing offers a versatile approach to correct, insert, or delete DNA sequences with exceptional accuracy and fewer unintended effects. Together, these methods represent critical advancements in gene editing, paving the way for novel therapeutic solutions to combat genetic diseases.
What is Base Editing?
Base editing was first introduced in 2016 by David Liu and his team as a new approach to precision gene editing. Unlike traditional CRISPR methods, which rely on creating double-strand breaks in DNA to achieve edits, base editing directly converts one DNA base into another through a deamination process. This process is achieved through the fusion of a CRISPR-Cas9 protein, programmed to target a specific DNA sequence, with a deaminase enzyme that facilitates the base conversion. The result is a highly precise edit at the target nucleotide without introducing a double-stranded break, reducing the risk of unintended genetic changes caused by insertions, deletions, and chromosomal translocation events.
The primary application of base editing lies in correcting point mutations, which account for a significant proportion of known genetic disorders. For instance, cytosine base editors (CBEs) convert cytosine (C) to thymine (T), while adenine base editors (ABEs) convert adenine (A) to guanine (G). By targeting these single nucleotide changes, base editing offers a powerful tool for studying gene function, developing disease models, and designing therapeutics for genetic diseases. With this, base editing offers precision and efficiency, making it an advantage over traditional CRISPR techniques.
Ongoing advancements in base editing focus on improving the accuracy, efficiency, and range of targetable sequences, as well as minimizing any off-target effects. Researchers are also exploring ways to enhance the delivery of base editors to specific cell types for therapeutic applications. For a deeper understanding of the science and therapeutic potential of base editing, please visit our CRISPR Base Editing Guide.
Check out our new Accubase™ Cytosine Base Editor available in Research-grade and GMP.
What is Prime Editing?
In October 2019, Andrew Anzalone, a postdoctoral fellow in David Liu's lab, and collaborators unveiled the groundbreaking development of prime editing in Nature. This innovative gene-editing technique enables precise small insertions, deletions, and base swaps, offering unparalleled precision. Prime editing may sound similar to traditional CRISPR systems at first glance, like deleting bases in genetic knockouts or inserting specific nucleotides in knock-ins. However, unlike traditional CRISPR systems, prime editing executes these changes without introducing double-stranded DNA breaks. Even more remarkable, targeted insertions are achieved without requiring donor DNA templates. Prime editing surpasses current base editing limitations, which are confined to just four possible base substitutions (C>T, G>A, T>C, A>G), by enabling all 12 possible nucleotide conversions.
Prime editing redefines the scope of gene editing by enhancing precision and flexibility. By overcoming the constraints of traditional CRISPR systems, prime editing provides a seamless, all-in-one solution that streamlines the editing process delivering impactful results. The use of prime editing is a pivotal step forward in genetic research and therapeutic applications, solidifying its role as a transformative gene editing tool for a variety of applications.
Components of CRISPR Prime Editing
Components of Prime Editing
Prime editing relies on a Cas endonuclease and a single guide RNA (sgRNA). However, it introduces key modifications to enable precise genome edits without creating double-strand breaks. Instead of using the standard Cas9 protein, prime editing uses a Cas9 nickase, a variant that nicks only one strand of the DNA. This Cas9 nickase is fused to a reverse transcriptase enzyme, forming what is known as a prime editor (PE). Reverse transcriptase, an enzyme commonly found in retroviruses, plays a crucial role in synthesizing DNA from an RNA template. Within the context of prime editing, reverse transcriptase uses the RNA sequence provided by the guide (pegRNA) to generate and insert the desired DNA changes directly into the genome. This innovative combination of Cas9 nickase and reverse transcriptase allows for seamless insertions, deletions, and base edits with minimal unintended effects.
The evolution of prime editors has seen significant advancements over time. PE1, the initial version, demonstrated the ability to perform small edits, including insertions, deletions, and base transversions, though with moderate editing efficiencies. To enhance performance, PE2 incorporated mutations to improve binding strength and thermostability, resulting in higher editing efficiencies. The latest iterations, PE3 and PE3b, expanded the capability of prime editing by addressing mismatch sequences that can occur during the editing process through the incorporation of a second guide RNA improving on its current precision and reliability [Anzalone et al., Nature (2019)].
What is the pegRNA?
The prime editing guide RNA (pegRNA) is a specialized guide RNA used exclusively in prime editing. It functions as a guide and a blueprint, directing the prime editor to the targeted DNA site while also encoding the instructions for the precise genetic modification to be made. Unlike traditional single guide RNAs (sgRNAs), pegRNAs are more complex and significantly longer due to the inclusion of additional sequence components essential for prime editing.
A pegRNA consists of four primary sequence parts:
- The target sequence, typically around 20 nucleotides, directs the Cas9 nickase to the specific DNA site for editing.
- The scaffold sequence that forms a secondary structure that binds Cas9 nickase, enabling it to function.
- The reverse transcription template sequence contains the desired edit and homology sequence. The typical size is from 25–40 nucleotides depending on the length of the desired genetic alteration.
- The primer-binding site (PBS), which is generally 10–15 nucleotides in length, serves as an anchor point for the reverse transcriptase to initiate DNA synthesis.
Altogether, the total length of a pegRNA generally falls between 120 and 145 nucleotides and can extend to 170-190 nucleotides or longer.
However, the extended length of pegRNAs brings unique challenges. First, synthesizing long RNA molecules with high fidelity can be technically demanding, increasing the potential for errors during production. Additionally, due to their size, pegRNAs can face lower yields during synthesis, challenges for purity analysis, and quality control due to potential secondary structures from complementary sequences. Finally, pegRNAs require careful optimization in delivery vectors such as plasmids or lipid nanoparticles due to the size of prime editors and pegRNA. Additionally, the delivery efficiency to cells can also be compromised since longer RNA sequences are more prone to degradation and can have reduced stability in cellular environments. These challenges necessitate ongoing innovations in pegRNA synthesis and delivery methods to maximize the effectiveness of prime editing in both research and therapeutic applications.
How Does Prime Editing Work?

Process of Prime Editing
Prime editing is a highly precise gene-editing technology that utilizes the PE:pegRNA complex to make targeted modifications to DNA. Below is a step-by-step explanation of how the process works:
Target Recognition and Binding
The prime editor (PE), which is a fusion of Cas9 nickase and reverse transcriptase, associates with the prime editing guide RNA (pegRNA) to form a complex. The pegRNA directs this complex to a specific DNA site by matching its target sequence to the complementary sequence on the DNA. This precise base-pairing ensures that only the intended site is targeted.
Creation of a Nicked Strand
Once bound to the target, the Cas9 nickase in the PE complex generates a single-strand cleavage or nick on one strand of the DNA, resulting in a loose single-stranded DNA flap. The 3’ end of the nicked strand becomes the starting point for editing. Unlike traditional CRISPR systems, no double-strand break is introduced, significantly reducing the risk of unintended edits and genomic instability.
Primer Binding and Reverse Transcription
The nicked DNA strand creates an opening for the primer-binding site (PBS), which is a sequence within the pegRNA, to anneal to the complementary region on the DNA flap starting on the 3’ end. At this point, the reverse transcriptase enzyme within the prime editor initiates the synthesis of the new DNA using the RNA sequence in the template region of the pegRNA. This newly generated DNA strand can then be incorporated into the growing DNA strand.
Insertion of Edited DNA and Flap Repair
The edited DNA strand synthesized by the reverse transcriptase is incorporated into the nicked DNA forming a 3’ flap competing with the unedited 5’ flap. Cellular repair mechanisms then process this structure, replacing the original sequence in the nicked DNA strand with the newly reverse-transcribed DNA carrying the desired edit. This step leaves one DNA strand edited and the complementary strand unedited, creating an intermediate state.
Correction of the Unedited Strand
To ensure both DNA strands carry the desired edit, the most recent iterations of the prime editing system, PE3 and PE3b, introduce a second guide RNA (gRNA). This additional gRNA directs the Cas9 nickase to create a nick on the unedited strand of DNA opposite the original edit. The edited DNA strand then serves as a repair template, ensuring the complementary strand is corrected to match the new sequence. This final step completes the prime editing process and creates a fully edited DNA duplex.
Removal of Original DNA Segment
During the repair process, the cellular endonuclease machinery removes leftover fragments of the original DNA that are no longer needed. By leveraging the precise nature of these endogenous mechanisms, prime editing seamlessly integrates the new genetic information into the genome.
Challenges with Prime Editing and their Solutions
Delivery Efficiency
Challenge: The large size of pegRNAs complicates their delivery into cells, and achieving efficient co-delivery of the Cas9 nickase-reverse transcriptase and pegRNA remains challenging.
Solution: Researchers are developing optimized delivery systems, such as lipid nanoparticles (LNPs), engineered viral vectors, and non-viral delivery approaches that accommodate the larger pegRNA size. Recent studies have shown success with dual-delivery strategies pairing LNPs with electroporation for improved cellular uptake. Engineering delivery vectors specifically tailored for pegRNAs has also minimized degradation and enhanced intracellular delivery efficiency.
Mismatch Repair and Reversal of Edits
Challenge: Cellular mismatch repair systems can reverse prime edits, lowering the overall efficacy of the approach.
Solution: The introduction of mismatch repair inhibitors, such as MLH1dn in the PE5 system, ensures that edits are retained by blocking cellular pathways that undo the modifications. Additionally, integrating damage-mitigating components directly into the prime editing machinery has improved the persistence and accuracy of edits. By refining the interaction of reverse transcriptase with the target DNA site, researchers have also reduced unintended cellular responses that compromise edits.
Potential Immune Responses
Challenge: The bacterial origin of Cas9 and RNA components could trigger immune responses, complicating therapeutic applications.
Solution: Researchers are developing Cas9 variants with reduced immunogenicity through directed evolution or engineering strategies. The use of transient delivery systems, such as non-integrative vectors or modified RNA delivery, minimizes prolonged exposure to bacterial components, reducing immune activation. Steps are also being taken to identify and genetically screen patients for predispositions to immune reactions, allowing pre-emptive mitigation strategies in therapeutic settings.
By actively addressing these challenges, prime editing continues to evolve, pushing the boundaries of its applications in precise, reliable, and scalable cell and gene therapies.
Advancements in Prime Editing Systems
Prime editing has progressed significantly, with each new variant enhancing efficiency, accuracy, and versatility. PE3 and PE3b marked early milestones by introducing methods to edit both DNA strands. PE3 employs an additional guide RNA to nick the unedited strand, allowing the newly edited strand to serve as a repair template, thereby improving overall editing efficiency. PE3b refines this system further by targeting secondary nicks closer to the original modification, minimizing off-target effects and boosting precision.
Recent advancements like PE5 take prime editing to new heights. By incorporating mismatch repair inhibitors such as MLH1dn, PE5 prevents cellular repair systems from reversing edits, ensuring the retention of intended modifications. With optimized guide RNA scaffolds and pegRNA designs, PE5 delivers enhanced binding stability and increased editing fidelity, making it highly effective for precise single-base substitutions and small fragment insertions. Additionally, advanced prime editor designs like PE4max and PE5max integrate dual nicking strategies and improved reverse transcriptase components, enabling large fragment edits while maintaining robust accuracy.
The development of the EXPERT (Extended Prime Editor System) marks a groundbreaking advancement in prime editing, significantly enhancing precision and versatility. By introducing extended pegRNAs (ext-pegRNAs) and an additional single-guide RNA (ups-sgRNA), EXPERT expands the editing range, enabling modifications on both sides of the DNA nick. This innovation makes complex genetic modifications, such as large-fragment edits or simultaneous multi-site edits, far more efficient, with improvements of up to 122-fold in editing efficiency compared to earlier systems like PE2.
EXPERT also prioritizes safety and precision through the use of paired cis nicks on the same DNA strand, reducing double-strand breaks and the risk of unintended large indels. This design results in high product purity with minimal off-target effects, ensuring genomic stability while achieving complex edits. What makes EXPERT even more versatile is its compatibility with other prime editor systems, such as PE3max and PE4, allowing researchers to adapt it to a variety of applications.
These advancements in prime editing, from the foundational innovations of PE3 and PE3b to the remarkable precision and efficiency of PE5 and EXPERT, collectively redefine what’s possible in genome editing. By addressing challenges like editing fidelity, off-target effects, and complex fragment modifications, these systems deliver unmatched versatility and reliability. Their transformative potential is already paving the way for breakthrough applications in both research and therapeutic development, establishing prime editing as a vital tool in tackling genetic disorders and advancing precision medicine.
Prime Editing Corrects Disease-Causing Mutations
PM359 Trial for Chronic Granulomatous Disease (CGD)
Prime Medicine's PM359 represents the first prime editing therapeutic candidate to enter clinical trials, marking a significant milestone in the field of genome editing. CGD, a rare inherited immunodeficiency, arises from mutations in genes encoding the NADPH oxidase complex, crucial for effective function of immune cells like neutrophils. These mutations impair pathogen-killing capabilities, leaving patients vulnerable to severe bacterial and fungal infections. While hematopoietic stem cell (HSC) transplants offer a potential cure, they pose risks such as graft-versus-host disease and require a suitable donor match, which can be challenging.
PM359 addresses these limitations by using ex vivo prime editing to correct mutations in the patient’s own HSCs. Specifically targeting the NCF1 gene, the most common CGD-associated mutation, this approach ensures a high percentage of corrected cells capable of repopulating the bone marrow and restoring immune function. Preclinical studies demonstrated robust engraftment and restored NADPH oxidase activity in edited cells. Currently, a global Phase 1/2 trial is underway to evaluate PM359's safety, biological activity, and preliminary efficacy, with initial results expected in 2025. The trial, which received rare pediatric and orphan drug designations, represents a novel therapeutic pathway for treating CGD with a highly targeted, precision-editing strategy.
Sickle Cell Disease and Tay-Sachs Proof-of-Concept Studies
Prime editing's potential to correct challenging genetic mutations has been demonstrated through proof-of-concept studies targeting sickle cell disease and Tay-Sachs disease. Sickle cell disease, caused by a single nucleotide mutation in the HBB gene, exemplifies prime editing's ability to perform precise single-base substitution edits with high efficiency. Similarly, Tay-Sachs disease, a lysosomal storage disorder resulting from a 4-bp insertion in the HEXA gene, highlights prime editing's capability to perform longer, more complex sequence corrections. Researchers successfully introduced and subsequently corrected these mutations in HEK293T cells using optimized pegRNAs, providing a clear demonstration of prime editing's versatility.
Early efforts to translate these approaches to clinically relevant systems also showed promise. For example, although modest (<10%) editing efficiency was achieved in post-mitotic cells derived from mice, these experiments underscored the need for advanced delivery methods to translate prime editing into therapeutic contexts. Lentiviral delivery was initially employed, but newer methods, such as lipid nanoparticle (LNP)-based systems, are being developed to enhance in vivo editing precision.
Expanding Therapeutic Applications
Beyond these initial demonstrations, prime editing is expanding to address a broader range of disease-causing mutations. By integrating advanced delivery platforms with improved editor designs, researchers are exploring treatments for diseases that require large-scale edits, such as cystic fibrosis and Duchenne muscular dystrophy. The safety profile of prime editing, which avoids double-strand DNA breaks, sets it apart from conventional CRISPR-Cas9 methods, reducing off-target effects and genomic instability.
Ongoing clinical trials like PM359 and the growing body of preclinical data underscore prime editing's transformative potential. By correcting mutations at the root cause of genetic diseases, these approaches signal a new era in precision medicine, offering hope for patients with genetic conditions that were previously considered untreatable. The field continues to evolve, with next-generation systems like EXPERT and enhanced pegRNA designs expected to further accelerate therapeutic development.
Is Gene Editing in Its Prime?
The advancements in prime editing signal a pivotal moment in the evolution of genome engineering, but it’s clear that this technology is still maturing. Recent breakthroughs, such as the PM359 clinical trial for chronic granulomatous disease, reflect its potential to transform treatments for genetic disorders. Yet, as promising as these innovations are, their application in the clinic is in its early stages. While initial studies have demonstrated success in commonly-used cell lines and select genetic diseases, the broader viability of prime editing in diverse, therapeutically relevant cell types, such as stem cells and primary cells, remains under investigation.
Ongoing research is critical to optimize the technique, enhance its precision, and fully assess its safety. Questions surrounding long-term effects, durability of edits, and the potential for off-target modifications must be resolved before prime editing can reach its full potential. Delivery methods are also an area of continuous improvement, as effective, reliable mechanisms for in vivo and ex vivo editing will be essential for advancing clinical applications. The active involvement of the research community, supported by the commercial availability of reagents and growing datasets from clinical trials, will play a vital role in addressing these challenges.
Despite these hurdles, the trajectory of prime editing is unmistakably forward. Each iteration of this technology not only improves its versatility and efficiency but also broadens its possible applications. Beyond correcting rare genetic diseases, prime editing opens doors to addressing complex conditions that were once considered out of reach for gene-based therapies. With continued collaboration, innovation, and rigorous testing, the dream of safely reversing genetic disorders may soon shift from an experimental possibility to a clinical reality. Gene editing may not yet be in its prime, but it is rapidly approaching that pinnacle, offering hope for a future where precision medicine transforms the lives of countless patients.