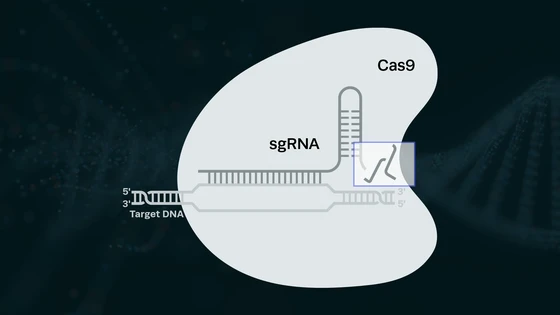
Malaria affects millions of people globally every year. With the introduction of CRISPR-Cas technology, several research groups are investigating genetically editing mosquitoes to reduce the occurrence of malaria.
In this article, we will cover the diverse projects wherein scientists are genetically editing mosquitoes using CRISPR to wipe out malaria and other vector borne diseases. Specially, we will learn about the following topics:
How can Genetically Modified Mosquitoes be used for Disease Control?
With changes brought about by international travel and climate change, mosquito borne diseases are no longer confined to tropical & subtropical countries. Outbreaks of Zika, malaria, and West Nile disease have become increasingly common in the United States. Scientists have concluded that the best way to eradicate these diseases is to attack the mosquito rather than the parasite. Recently, scientists have turned their attention to genetic engineering as an approach to control mosquito population.
Two approaches are currently being used to control mosquito-borne diseases using gene-edited mosquitoes. The first approach is "population replacement", where a mosquito population that can transmit pathogens is replaced by one that is unable to do so. In order to spread the anti-pathogen genes using this method, scientists rely on a concept known as the gene drive. Gene drive works by using a quirk in inheritance to spread a certain trait to more than half of a mosquito's offspring, hence spreading rapidly through a population.
The other strategy is known as "population suppression". This approach uses gene editing to reduce mosquito populations so that there are fewer mosquitoes to pass on the pathogen.
In the following sections, we will learn how these two approaches are being used to eradicate malaria, and specifically how CRISPR- a novel genome editing tool has helped scientists make dramatic progress in controlling the spread of this disease.
CRISPR Gene Drive: Mosquito Eradication Could Be Possible
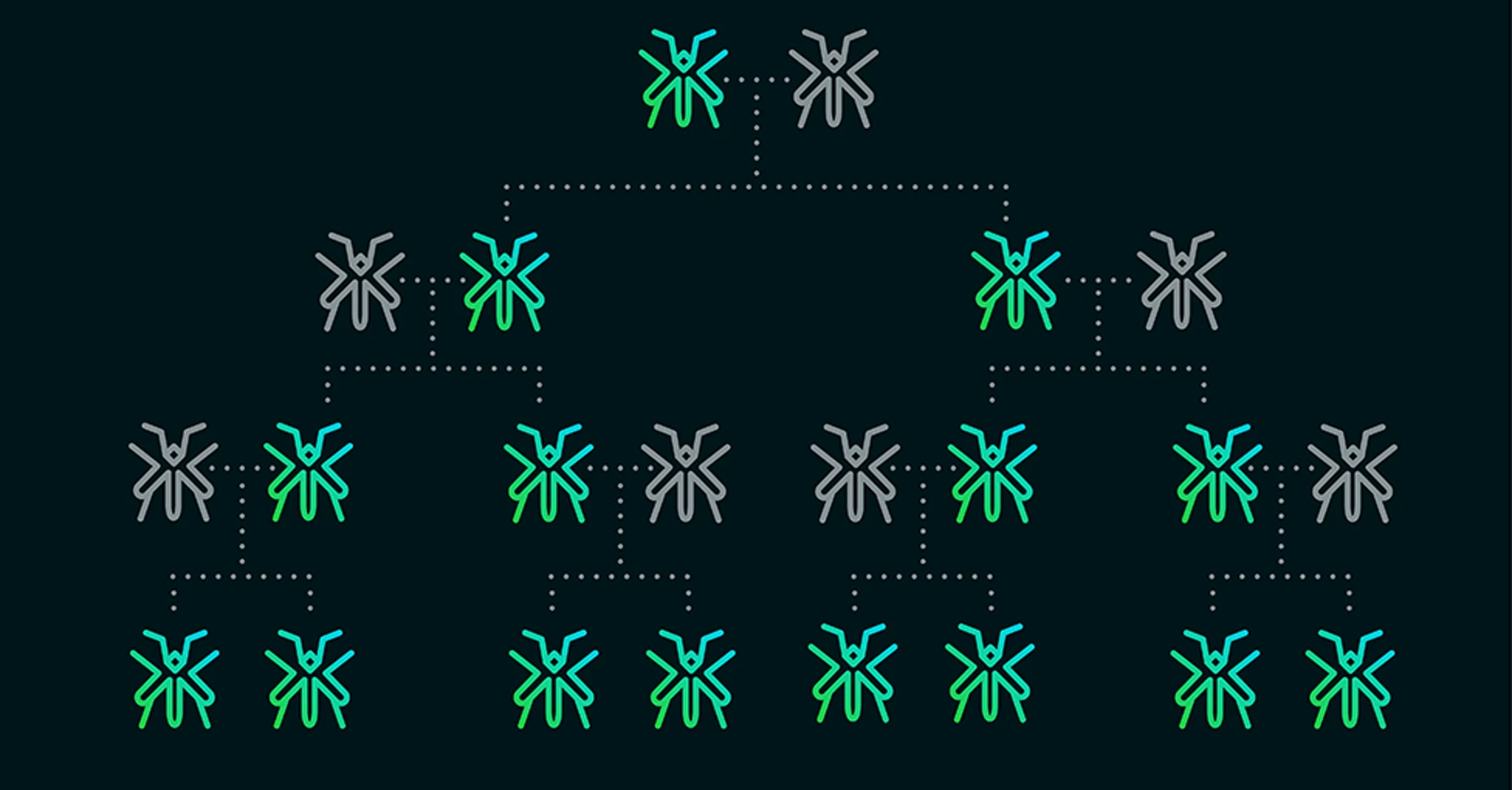
As an approach to control mosquito population, recently scientists have explored the idea of using CRISPR in gene drive—self-propagating genetic elements that can rapidly spread through a population. Gene drives in mosquitoes could be used to combat malaria in several ways. For instance, a gene drive could spread a gene in the mosquito population that prevents transmission of the malaria parasite. Alternatively, they could be used to dramatically reduce the size of a mosquito population altogether.
Now, the potential for use of gene drives to eradicate an entire population of mosquitoes seems much more real. A team of researchers from Imperial College London led by Professor Andrea Crisanti did just that.
Crisanti and his team used CRISPR to modify the sex-determining gene, making male gene dominant over the female one. The modification was then spread using a gene drive, with the expectation that females would eventually be eliminated from the population. Only female mosquitoes are able to transmit malaria, so eradicating females would mean that malaria would be transmitted to humans less often.
Initially, 150 male mosquitoes carrying the modified gene drive were released in the cage. The modified gene reached 100% prevalence within 7-11 generations, progressively reducing egg production to the point of total population collapse. It is noteworthy that this experiment was undertaken within a laboratory environment. Further research and field testing be conducted before any genetically engineered mosquitoes are released into the wild.
CRISPR Mosquito: Gene Knockout Creates Malaria Resistant Population

Plasmodium relies on several agonists as it travels through the mosquito, and by inhibiting or these agonists pre- or post-transcriptionally, transmission-blocking can be achieved. The advent of CRISPR-Cas9 based tools have provided scientists with many opportunities to study the agonist function and develop disease control strategies. A team of scientists from John Hopkins University were first to show that deleting a gene from mosquitoes can make them resistant to malaria parasites.
This group investigated the effect of fibrinogen-related protein 1 (FREP1) gene inactivation using CRISPR technology. FREP1 had previously been investigated by using RNAi-mediated gene silencing methodology. However, since RNAi-based gene silencing results in only partial protein depletion, FREP1 silencing resulted in only an 11% decrease in P. falciparum infection prevalence. CRISPR, on the other hand can be used to completely knock out the gene, and in the current study, they used it to disrupt the FREP1 gene.
Using CRISPR technology, scientists were able to show that FREP1 knockout mosquitoes exhibited a slower pre-adult development, were less likely to feed on blood meals when given the opportunity and laid fewer and less viable eggs when compared to their wild type counterparts.
Cutting Back Malaria: CRISPR-Cas9 Gene-Editing Implications
Apart from the studies discussed above, there have been many other studies using CRISPR as a tool to genetically engineer the Plasmodium species—the organism responsible for causing malaria. A review article published in Briefings in Functional Genomics, summarizes all the studies that have used CRISPR to modify Plasmodium.
CRISPR certainly has the potential to control or even wipe out mosquito populations. However, before doing so, scientists must carefully weigh the ethical implications of using this tool at a species level. The impact on biodiversity and ecosystems must be carefully considered before drastically modifying mosquito population.
The debate on the ethics of fighting malaria with CRISPR-Cas9 will continue on for a while.
Gene Knockouts
Gene knockouts, in which genes are inactivated so that they no longer make functional protein, are used to address a variety of scientific questions. The CRISPR gene editing tool has made knocking out genes easier than ever before.