Contents
Do you know what connects “Back to the Future” actor Michael J. Fox, boxing champion Muhammad Ali, folk-rock sensation from the 60’s Linda Ronstadt, and the former NBA fame Brian Grant? They are all noted celebrities diagnosed with Parkinson’s disease. A debilitating neurological condition with no known cure, Parkinson’s can affect people from all walks of life.
As we observe National Parkinson’s Awareness Month this April, let’s learn more about the biology of Parkinson’s disease, current treatment options, and the progress towards finding a future cure. We will discuss in specific detail the recent developments in CRISPR-based research in the field. We will see how CRISPR creates avenues for multiple clinical applications and possible therapies in Parkinson’s through its precision, efficacy, and adaptability.
Parkinson’s Disease: Causes, Symptoms, & Diagnosis
Parkinson’s Disease is a common neurodegenerative disorder, second only to Alzheimer’s Disease. In a study published in 2022 in the NPJ Parkinson’s Disease Journal, researchers estimated that nearly 90,000 new cases of Parkinson's are diagnosed annually in the United States in individuals aged 65 and older. This is a steep 50% increase compared to the estimate from the previous years. Worldwide, the prevalence is estimated to be around 10 million people. The average age at diagnosis is 60 years, and it is primarily an age-related condition. With more aging populations being affected, Parkinson's puts a substantial socioeconomic burden on healthcare systems and caregivers.
The biology behind Parkinson's diseaseParkinson's affects a particular type of neuron called dopaminergic neuron. These neurons are found in the substantia nigra region of the midbrain and are essential for voluntary movement and behavioral processes. Neurons communicate by releasing specific chemical signals called neurotransmitters. Dopaminergic neurons produce large amounts of a neurotransmitter called dopamine. As these neurons degenerate in individuals with Parkinson's with time, dopamine production declines, causing motor dysfunction.
Aggregates of misfolded ɑ-synuclein protein, also known as Lewy bodies, are common pathological determinants in Parkinson's. Mounting evidence now suggests that Lewy bodies may be involved in the underlying pathophysiology of Parkinson’s. Learn more about the cellular biology of Parkinson's in this video.
Parkinson's affects a particular type of neuron called dopaminergic neuron. These neurons are found in the substantia nigra region of the midbrain and are essential for voluntary movement and behavioral processes. Neurons communicate by releasing specific chemical signals called neurotransmitters. Dopaminergic neurons produce large amounts of a neurotransmitter called dopamine. As these neurons degenerate in individuals with Parkinson's with time, dopamine production declines, causing motor dysfunction.
Aggregates of misfolded ɑ-synuclein protein, also known as Lewy bodies, are common pathological determinants in Parkinson's. Mounting evidence now suggests that Lewy bodies may be involved in the underlying pathophysiology of Parkinson’s. Learn more about the cellular biology of Parkinson's in this video.
What causes Parkinson’s is not very well understood. Only a subset of the cases (up to 10%) is genetically inherited. The vast majority, however, occur sporadically.

Parkinson's disease symptomsParkinson's is unique for every patient, and symptoms during early onset can be elusive. Tremors at rest, slow and rigid movement (bradykinesia), hunched posture, and unbalanced gait are primary motor symptoms associated with the disorder. Many patients experience a slurred, soft speech, loss of smell, and cognitive impairment.
Additional signs that may well precede the medical diagnosis include depression, anxiety, and sleep disorders. In most cases, a primary care provider performs the initial diagnosis, suggesting further consultation with a “movement disorder specialist,” a neurologist specializing in Parkinson's diagnosis.
We do not understand much about what causes the disease, except that it may be an interplay of genetic and environmental factors in some cases. Having an affected family member can increase the risk associated, but the familial risk is only in up to 10% of all the patients. In most cases, the diagnosis is spontaneous and may even be unwarned!
There are no known cures for Parkinson's, and scientists are looking for solutions to cope with the disease’s debilitating effects. Early detection and identification of biomarkers is, therefore, key to the holistic management of Parkinson's.
Parkinson's is unique for every patient, and symptoms during early onset can be elusive. Tremors at rest, slow and rigid movement (bradykinesia), hunched posture, and unbalanced gait are primary motor symptoms associated with the disorder. Many patients experience a slurred, soft speech, loss of smell, and cognitive impairment.
Additional signs that may well precede the medical diagnosis include depression, anxiety, and sleep disorders. In most cases, a primary care provider performs the initial diagnosis, suggesting further consultation with a “movement disorder specialist,” a neurologist specializing in Parkinson's diagnosis.
We do not understand much about what causes the disease, except that it may be an interplay of genetic and environmental factors in some cases. Having an affected family member can increase the risk associated, but the familial risk is only in up to 10% of all the patients. In most cases, the diagnosis is spontaneous and may even be unwarned!
There are no known cures for Parkinson's, and scientists are looking for solutions to cope with the disease’s debilitating effects. Early detection and identification of biomarkers is, therefore, key to the holistic management of Parkinson's.
Parkinson's diagnosis and testingConventionally, Parkinson's diagnosis is confirmed solely based on the four primary motor symptoms mentioned above, with the affected individual presenting with at least two out of the four symptoms. This means most of the neurodegeneration has already set in by the time a patient is diagnosed.
In a groundbreaking study recently published in The Lancet Neurology, scientists have revealed a new tool to detect a Parkinson’s biomarker in individuals who have not been diagnosed and may even be asymptomatic but at a high risk of developing the disease. The study was conducted on a large and multicenter cohort of individuals through the Parkinson’s Progression Markers Initiative (PPMI).
Alpha-synuclein (ɑ-synuclein) protein folds to form aggregates in Parkinson’s patients and is detectable in the cerebrospinal fluid (CSF). The novel method known as ɑ-synuclein amplification assay (αSyn-SAA) is based on the fluorescent detection of folded ɑ-synuclein protein. The results from the study conducted on 1,123 individuals showed remarkably accurate detection of abnormal protein in 93 percent of participants with Parkinson’s. This is a significant step in the early detection of Parkinson’s which is critical for disease management. This study was funded by the Michael J. Fox Foundation. The next steps in the assay development would be to optimize it for quantitation and to be able to detect markers through a simple blood draw or nasal swab test instead of a painful spinal tap.
In another study published a couple of years ago in Nature Communications, researchers in the United Kingdom, in collaboration with Joy Milne, a retired nurse, reported a potential skin swab test to detect Parkinson's precisely. The test utilizes a mass-spectrometry-based analysis of skin swab samples. It analyzes secreted components from dermal oil glands, also known as sebaceous glands. Once approved, it will prove to be a fast, painless, readily accessible, and definitive test to diagnose Parkinson's Disease.
Joy's late husband Les was diagnosed with Parkinson's while still in his early 40s. Joy has a rare ability to detect Parkinson’s through smell long before an actual clinical diagnosis and is now helping individuals at high risk through early detection.
Conventionally, Parkinson's diagnosis is confirmed solely based on the four primary motor symptoms mentioned above, with the affected individual presenting with at least two out of the four symptoms. This means most of the neurodegeneration has already set in by the time a patient is diagnosed.
In a groundbreaking study recently published in The Lancet Neurology, scientists have revealed a new tool to detect a Parkinson’s biomarker in individuals who have not been diagnosed and may even be asymptomatic but at a high risk of developing the disease. The study was conducted on a large and multicenter cohort of individuals through the Parkinson’s Progression Markers Initiative (PPMI).
Alpha-synuclein (ɑ-synuclein) protein folds to form aggregates in Parkinson’s patients and is detectable in the cerebrospinal fluid (CSF). The novel method known as ɑ-synuclein amplification assay (αSyn-SAA) is based on the fluorescent detection of folded ɑ-synuclein protein. The results from the study conducted on 1,123 individuals showed remarkably accurate detection of abnormal protein in 93 percent of participants with Parkinson’s. This is a significant step in the early detection of Parkinson’s which is critical for disease management. This study was funded by the Michael J. Fox Foundation. The next steps in the assay development would be to optimize it for quantitation and to be able to detect markers through a simple blood draw or nasal swab test instead of a painful spinal tap.
In another study published a couple of years ago in Nature Communications, researchers in the United Kingdom, in collaboration with Joy Milne, a retired nurse, reported a potential skin swab test to detect Parkinson's precisely. The test utilizes a mass-spectrometry-based analysis of skin swab samples. It analyzes secreted components from dermal oil glands, also known as sebaceous glands. Once approved, it will prove to be a fast, painless, readily accessible, and definitive test to diagnose Parkinson's Disease.
Joy's late husband Les was diagnosed with Parkinson's while still in his early 40s. Joy has a rare ability to detect Parkinson’s through smell long before an actual clinical diagnosis and is now helping individuals at high risk through early detection.
Parkinson’s Disease Treatment and Research: CRISPR As An Emerging Tool
There is no cure for Parkinson's, and treatment is mainly symptomatic, meaning the therapies available today can only help manage the condition. Levodopa, the most frequently prescribed Parkinson's medication, is a dopamine precursor and crosses the blood-brain barrier (BBB) to produce dopamine inside the brain.
Earlier this year, results from the Phase III BouNDless trial using ND0612, a liquid form of Levodopa/ Carbidopa that is infused subcutaneously, showed ND0612 to be more effective compared to oral LD/CD pills. This novel liquid drug is on the way for approval by the U.S. Food and Drug Administration (FDA). The advantage of having a liquid drug that can be administered 24 hours a day under the skin is that it is homogeneously absorbed in Parkinson’s patients compared to oral pills that may not be regularly absorbed by the digestive tract.
Dopamine agonists are also used in first-line therapy for Parkinson's patients. Surgery and deep brain stimulation may be effective in later stages when patients develop resistance to medications. Although effective, surgeries can lead to serious side effects, including bleeding in the brain.
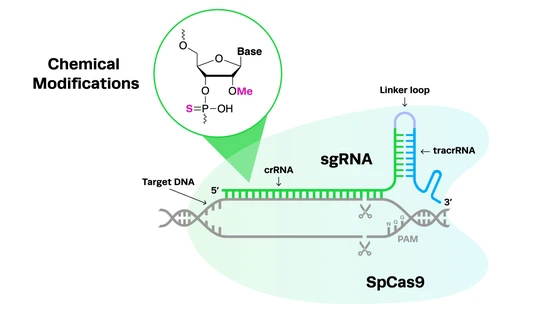
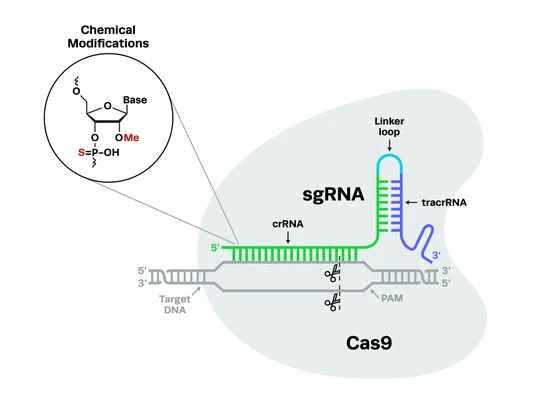
Familial Parkinson's (inherited genetically) involves both autosomal recessive and dominant patterns of inheritance. However, genetic variants linked to most sporadic Parkinson's occurrences are unclear. Similarly, molecular mechanisms of Parkinson's progression are yet to be completely deciphered. The challenge with studying the underlying mechanisms of Parkinson's is the complexity of multiple genetic mutations that may be involved. Given the multitude, CRISPR-Cas9 can be well-utilized to screen genetic variants in Parkinson's, particularly functional screens, to determine causality. CRISPR-Cas9 has been and can further be effectively used to develop cellular and whole-organism research models to study Parkinson's phenotype.
Genetic mutations in Parkinson's and the CRISPR solutionCRISPR screening uses CRISPR as a tool to identify genes or genetic sequences governing specific morphological or physiological effects. CRISPR screening is vital to understanding genes associated with Parkinson's mechanisms and targeting them for future therapy.
Scientists have long known that some neuronal cells are more susceptible to damage with aging and neurodegeneration. This phenomenon, also known as selective neuronal vulnerability, is reported in Parkinson's and other neurological conditions such as Alzheimer’s. Conventionally, gene expression studies (transcriptome and proteome) have revealed the differences in vulnerable versus resilient neurons. However, genetic screens are essential to understand the molecular mechanisms at a functional level and therapeutically target relevant genes.
CRISPR can be effectively used to study gene function in neuronal cell models. A pertinent example of one such study comes from the Hoffman lab at the University of Pittsburgh, GA, that leveraged Synthego’s genome editing potential to generate NADPH oxidase (NOX1, NOX2, and NOX4) knockout cell models for Parkinson’s. The team published their findings in the Neurobiological Diseases Journal in August 2022. The study demonstrated the role of the Nox2 enzyme in oxidative stress-related degeneration, including ɑ-synuclein accumulation, protein import impairment in mitochondria, and the activation of leucine-rich repeat kinase 2 (LRRK2).
CRISPR screening uses CRISPR as a tool to identify genes or genetic sequences governing specific morphological or physiological effects. CRISPR screening is vital to understanding genes associated with Parkinson's mechanisms and targeting them for future therapy.
Scientists have long known that some neuronal cells are more susceptible to damage with aging and neurodegeneration. This phenomenon, also known as selective neuronal vulnerability, is reported in Parkinson's and other neurological conditions such as Alzheimer’s. Conventionally, gene expression studies (transcriptome and proteome) have revealed the differences in vulnerable versus resilient neurons. However, genetic screens are essential to understand the molecular mechanisms at a functional level and therapeutically target relevant genes.
CRISPR can be effectively used to study gene function in neuronal cell models. A pertinent example of one such study comes from the Hoffman lab at the University of Pittsburgh, GA, that leveraged Synthego’s genome editing potential to generate NADPH oxidase (NOX1, NOX2, and NOX4) knockout cell models for Parkinson’s. The team published their findings in the Neurobiological Diseases Journal in August 2022. The study demonstrated the role of the Nox2 enzyme in oxidative stress-related degeneration, including ɑ-synuclein accumulation, protein import impairment in mitochondria, and the activation of leucine-rich repeat kinase 2 (LRRK2).
CRISPRi-based screening in neurodegenerative disorders
Researchers from the Kampmann lab at the University of California, San Francisco, have used CRISPR interference (CRISPRi) tools to develop functional genetic screens in induced pluripotent stem cells (iPSC)-derived human neurons. CRISPRi has significantly fewer off-target effects compared to conventional RNAi screens. CRISPRi-mediated partial knockdown enables investigating essential genes at varying expression levels via variable degrees of knockdown. Similar platforms can be designed to understand the molecular mechanisms underlying specific neurobiologies. Eventually, the goal is to identify potential therapeutic targets for multiple neurodegenerative conditions.

The 2P’s of Parkinson’s: PINK1 and PRKNNeurodegeneration in Parkinson's is characterized by inefficient mitophagy, redox metabolism, and aberrant mitochondria. Mitochondria are membrane-bound cell organelles also nicknamed “powerhouse of the cell.” They supply energy to ensure healthy cell functioning and maintenance. Cells with disintegrated and dysfunctional mitochondria use mitophagy as a self-cleansing mechanism. Not surprisingly, aberrant mitochondrial pathways have been linked to neurodegeneration in Parkinson's.
PTEN-induced Putative Kinase 1 (PINK1) and Parkin (E3 ubiquitin ligase) proteins work hand in hand through common pathways driving mitochondrial turnover and mitophagy. PINK1 is a serine/threonine kinase that phosphorylates mitochondrial proteins and protects mitochondria during cellular stress. Parkin is a ubiquitin ligase that tags mitochondrial proteins for mitophagy. The PINK1 and PRKN genes encode for PINK1 and Parkin proteins, respectively. Both these genes are often mutated in Parkinson’s disease.
Evidence from studies in PINK1-/- mice models, published in Nature Letters, suggest an immunosuppressive role of the protein, especially in the context of gut bacterial infections. Pathophysiological data from the study points to the fact that gut infections can trigger Parkinson's like symptoms in these animal models.
Neurodegeneration in Parkinson's is characterized by inefficient mitophagy, redox metabolism, and aberrant mitochondria. Mitochondria are membrane-bound cell organelles also nicknamed “powerhouse of the cell.” They supply energy to ensure healthy cell functioning and maintenance. Cells with disintegrated and dysfunctional mitochondria use mitophagy as a self-cleansing mechanism. Not surprisingly, aberrant mitochondrial pathways have been linked to neurodegeneration in Parkinson's.
PTEN-induced Putative Kinase 1 (PINK1) and Parkin (E3 ubiquitin ligase) proteins work hand in hand through common pathways driving mitochondrial turnover and mitophagy. PINK1 is a serine/threonine kinase that phosphorylates mitochondrial proteins and protects mitochondria during cellular stress. Parkin is a ubiquitin ligase that tags mitochondrial proteins for mitophagy. The PINK1 and PRKN genes encode for PINK1 and Parkin proteins, respectively. Both these genes are often mutated in Parkinson’s disease.
Evidence from studies in PINK1-/- mice models, published in Nature Letters, suggest an immunosuppressive role of the protein, especially in the context of gut bacterial infections. Pathophysiological data from the study points to the fact that gut infections can trigger Parkinson's like symptoms in these animal models.
Non-human primate model using CRISPR-mediated PINK1 deletionScientists used CRISPR/Cas9-mediated PINK1 deletion to generate a Rhesus macaque model, recapitulating the human Parkinson's phenotype. Remarkably, they observed neuronal loss in the substantia nigra region in the midbrain of affected animals (a common observation in Parkinson's patients). CRISPR-Cas9 mosaicism enabled different degrees of PINK1 deletion, allowing researchers to understand the complexity of the associated phenotypes.
Scientists used CRISPR/Cas9-mediated PINK1 deletion to generate a Rhesus macaque model, recapitulating the human Parkinson's phenotype. Remarkably, they observed neuronal loss in the substantia nigra region in the midbrain of affected animals (a common observation in Parkinson's patients). CRISPR-Cas9 mosaicism enabled different degrees of PINK1 deletion, allowing researchers to understand the complexity of the associated phenotypes.
CRISPR-based genetic screens to identify Parkin regulatorsScientists used a genome-wide CRISPR screen to identify both positive and negative regulators of Parkin expression successfully. Parkin protein abundance has been associated with Parkinson’s disease, but what regulates the protein abundance is not well understood. The study used a CRISPR-based functional genetic screen to identify transcriptional regulators of Parkin abundance. Similar CRISPR-based screens can identify other novel regulators of the PRKN/PINK1 axis to understand the pathways better.
Scientists used a genome-wide CRISPR screen to identify both positive and negative regulators of Parkin expression successfully. Parkin protein abundance has been associated with Parkinson’s disease, but what regulates the protein abundance is not well understood. The study used a CRISPR-based functional genetic screen to identify transcriptional regulators of Parkin abundance. Similar CRISPR-based screens can identify other novel regulators of the PRKN/PINK1 axis to understand the pathways better.
The alpha factor: alpha-synuclein in Parkinson’sAlpha-synuclein (ɑ-synuclein) protein is abundant in dopaminergic neurons and is encoded by the SNCA gene. The protein’s normal function is not very well understood and has a suggested presynaptic role. Insoluble aggregates of misfolded ɑ-synuclein protein form lesions called Lewy bodies, a distinct pathologic feature in Parkinson's-affected dopaminergic neurons.
Mutations and multiplications in the SNCA gene have been linked to Parkinson's development and progression.
Alpha-synuclein (ɑ-synuclein) protein is abundant in dopaminergic neurons and is encoded by the SNCA gene. The protein’s normal function is not very well understood and has a suggested presynaptic role. Insoluble aggregates of misfolded ɑ-synuclein protein form lesions called Lewy bodies, a distinct pathologic feature in Parkinson's-affected dopaminergic neurons.
Mutations and multiplications in the SNCA gene have been linked to Parkinson's development and progression.
Potential therapeutic screen for Parkinson’s using CRISPRResearchers developed a novel platform using CRISPR-Cas9 to modify the SNCA gene with a bioluminescent tag and measure ɑ-synuclein protein generation. The luminescent signal generated with ɑ-synuclein expression is the measurable readout. This was the first quantitative system to detect endogenous ɑ-synuclein (with no external reporters) and has potential implications as a high-throughput drug screening tool for Parkinson's.
Researchers developed a novel platform using CRISPR-Cas9 to modify the SNCA gene with a bioluminescent tag and measure ɑ-synuclein protein generation. The luminescent signal generated with ɑ-synuclein expression is the measurable readout. This was the first quantitative system to detect endogenous ɑ-synuclein (with no external reporters) and has potential implications as a high-throughput drug screening tool for Parkinson's.
Altering the epigenetic landscape with CRISPRIn addition to the genetic multiplication of SNCA, changes in the epigenetic landscape and the resulting regulation of SNCA gene expression can lead to excess ɑ-synuclein protein in the neurons. Histone modifications, especially histone methylation, are common epigenetic alterations.
In a study published by the Kim lab at the University of Central Florida, researchers adopted a CRISPR-Cas9 approach to understanding the epigenetic regulation of the SNCA gene. Using a histone demethylase as an “epigenetic eraser,” they identified a specific histone modification regulating the SNCA gene. The system was developed using neurons from patient-derived stem cells. Identifying epigenetic regulators of SNCA opens up new avenues for potential therapeutic targeting in Parkinson's.
In addition to the genetic multiplication of SNCA, changes in the epigenetic landscape and the resulting regulation of SNCA gene expression can lead to excess ɑ-synuclein protein in the neurons. Histone modifications, especially histone methylation, are common epigenetic alterations.
In a study published by the Kim lab at the University of Central Florida, researchers adopted a CRISPR-Cas9 approach to understanding the epigenetic regulation of the SNCA gene. Using a histone demethylase as an “epigenetic eraser,” they identified a specific histone modification regulating the SNCA gene. The system was developed using neurons from patient-derived stem cells. Identifying epigenetic regulators of SNCA opens up new avenues for potential therapeutic targeting in Parkinson's.
Dangers lurking in LRRK2 mutationsLeucine-rich repeat kinase 2 (LRRK2) gene encodes for a kinase called dardarin and is active in the brain and other tissues. LRRK2 mutations cause morphological and physiological changes in Parkinson's-affected neurons in mice. A single mutation G2019S in the kinase domain of LRRK2 is the most commonly found (present in nearly 1-3% of Parkinson's patients worldwide). G2019S mutation causes mitochondrial damage and calcium dysregulation in iPSC-derived neurons from Parkinson's patients.
The Cookson lab at the National Institute of Health, MD, published their study on isogenic cell lines for the LRRK2 gene a couple of years ago. These cell lines could prove to be effective models for studying Parkinson’s disease.
Leucine-rich repeat kinase 2 (LRRK2) gene encodes for a kinase called dardarin and is active in the brain and other tissues. LRRK2 mutations cause morphological and physiological changes in Parkinson's-affected neurons in mice. A single mutation G2019S in the kinase domain of LRRK2 is the most commonly found (present in nearly 1-3% of Parkinson's patients worldwide). G2019S mutation causes mitochondrial damage and calcium dysregulation in iPSC-derived neurons from Parkinson's patients.
The Cookson lab at the National Institute of Health, MD, published their study on isogenic cell lines for the LRRK2 gene a couple of years ago. These cell lines could prove to be effective models for studying Parkinson’s disease.
LRRK2 mutant Marmoset neuronal cell model using CRISPRIn a 2020 study published from the Golos lab at the University of Wisconsin-Madison, scientists created neuronal cell models with LRRK2 G2019S mutation developed from marmoset-derived stem cells. The resulting cell models closely mimic the human dopaminergic neuron physiology and present an excellent opportunity to explore the natural disease progression.
In a 2020 study published from the Golos lab at the University of Wisconsin-Madison, scientists created neuronal cell models with LRRK2 G2019S mutation developed from marmoset-derived stem cells. The resulting cell models closely mimic the human dopaminergic neuron physiology and present an excellent opportunity to explore the natural disease progression.
Gene Therapy and Clinical Trials in Parkinson’s

Gene therapy involves techniques that manipulate human genes to treat or cure diseases and holds tremendous potential to cure several genetic disorders. In January 2021, the first-ever gene-edited cell therapy for Parkinson's entered a Phase I clinical trial. The US FDA approved an Investigational New Drug (IND) application from BlueRock Therapeutics in collaboration with the Memorial Sloan Kettering Cancer Center.
The phase I trial will test the safety and toxicity of human embryonic stem cell (hESC)-derived cells called MSK-DA01 transplanted in Parkinson's patients. Gene-editing equips the cells for immune evasion post-transplantation in patients.
In June 2022, Blue Rock Therapeutics completed the full enrolment for the trial, recruiting up to 12 people in the U.S. and Canada between ages 50-76. These individuals had been on Levodopa for over a decade and are no longer fully responsive to the drug. The FDA also approved a fast-track designation for DA01. The company expects to announce results from Phase I around the second half of 2023.
A preclinical study using MSK-DA01 cells demonstrated both safety and efficacy in rat midbrains. The cells, when transplanted, produced dopamine and rescued Parkinson's motor dysfunction. Rigorous testing for tumorigenicity, toxicity, biodistribution, and other adverse effects did not reveal any concerns.
More recently, physicians and scientists at the West Virginia Rockefeller Neuroscience Institute initiated a clinical trial to evaluate the safety and efficacy of an experimental gene therapy, AAV-GAD, in collaboration with MeiraGTx, a clinical-stage gene therapy company. The AAV-GAD gene therapy is delivered surgically into an affected area in the brain, called the subthalamic nucleus, and does not require any cell implants. The first patient enrolled in this trial underwent surgery in December 2022.
There have been numerous pre-clinical studies and some clinical trials using the gene therapy approach in Parkinson’s with varying degrees of success. Currently, scientists and physicians continue to harness gene therapy to possibly cure Parkinson's with an impressive list of late-stage clinical trials.
Future Possibilities With CRISPR in Parkinson’s Disease
With the overwhelming success of CRISPR-Cas gene-editing systems in driving clinical research, there is immense hope for applications in Parkinson's and other neurodegenerative diseases. As with other novel therapeutic approaches, the scientific and medical communities continue to be vigilant of the related ethical concerns (an interesting parallel could be drawn from concerns around regenerative cell therapy in Parkinson's).
The Innovative Genomics Institute (IGI) at Berkeley, the University of California, San Francisco, and Genentech have now joined hands to form the Alliance for Therapies in Neuroscience (ATN). ATN is a long-term research partnership involving cross-collaboration spanning multiple disciplines, such as CRISPR technology, clinical neuroscience, and biophysics. The overarching goal of this collaboration is to harness the power of CRISPR technology to study complex genetic interactions and molecular mechanisms underlying neurodegenerative disorders such as Parkinson’s disease and hopefully treat them. Watch this video to learn more about the program and to hear from Dr. Jennifer Doudna and other collaborators.
Although the future remains to be seen, there is growing optimism for CRISPR-based clinical advancements in Parkinson’s Disease.