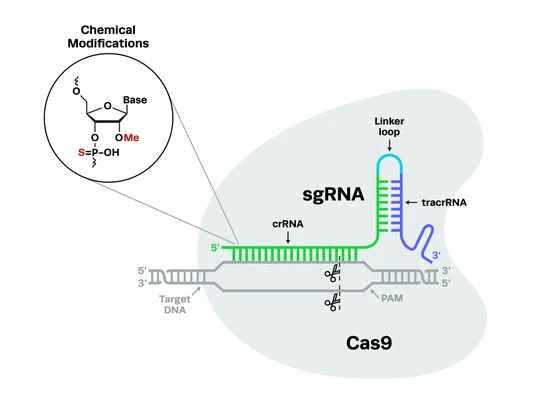
Contents
With nearly 44 million people suffering from Alzheimer’s or related dementia, this disease is one of the primary afflictions in older individuals. Alzheimer’s disease was first discovered over a century ago, but to date, there is no cure. With the high failure rate of pharmaceutical clinical trials for Alzheimer’s treatment, there is a greater need for alternate strategies. Recently, with the discovery of CRISPR editing, there has been hope that this tool will help shed light on the pathogenesis of the disease and possibly provide therapeutic options.
What is Alzheimer’s Disease?
Alzheimer’s disease (AD) is an irreversible and progressive neurological disorder that leads to cognitive decline. It is the leading cause of dementia and some of the associated symptoms are memory and orientation loss combined with other cognitive impairments. The disease is named after Dr. Alois Alzheimer who discovered it in 1906.
The majority of the people affected are 65 and older, with increasing age being the primary risk factor for Alzheimer’s. Currently, it affects over 5.5 million people in the United States alone and this number is expected to triple by 2050 (1). Although research has been ongoing for decades, scientists have not found a cure yet. Most of the present treatments can only slow the dementia symptoms from worsening but cannot stop disease progression.
What causes Alzheimer’s?While scientists haven’t fully understood the exact causes of the disease yet, they believe that a combination of genetic, lifestyle, and environmental factors might play a role. Advances in brain imaging technologies have allowed researchers to identify the role of two abnormal structures called plaques and tangles in neurodegeneration, which leads to memory loss. Scientists have also found several gene mutations that cause the disease to be passed on from parent to child.
The key to understanding whether Alzheimer’s is hereditary or not lies in the genetics of the disease. Genes encoding four main proteins have been identified so far in association with AD, amyloid precursor protein (APP), presenilin 1 (PSEN1), presenilin 2 (PSEN2), and apolipoprotein E (APOE). The first three gene mutations have been linked to early-onset (familial) Alzheimer’s disease, whereas the fourth one is considered to be a major risk factor in the late-onset of the disease.
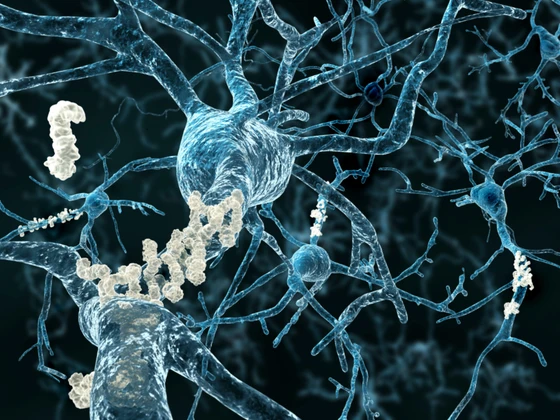
The scientists showed in the early 1980s that an AD patient’s brain is characterized by an extensive distribution of two types of abnormal structures: senile plaques and neurofibrillary tangles (NFTs) (3). This led them to propose two main hypotheses to describe AD pathogenesis: the amyloid hypothesis and the tau hypothesis.
Senile plaques typically consist of a core of amyloid fibrils composed of the amyloid β (Aβ) peptide and are surrounded by degenerating neurons. According to the amyloid hypothesis, the production of Aβ in the brain initiates a series of events causing the clinical syndrome of dementia. Meanwhile, the tau hypothesis states that the hyperphosphorylation of tau is the primary cause of neurodegeneration in AD. NFT’s are composed of bundles of phosphorylated tau proteins. When tau is hyperphosphorylated, it dissociates from microtubules and aggregates into NFTs contributing to neural damage (4).
While scientists haven’t fully understood the exact causes of the disease yet, they believe that a combination of genetic, lifestyle, and environmental factors might play a role. Advances in brain imaging technologies have allowed researchers to identify the role of two abnormal structures called plaques and tangles in neurodegeneration, which leads to memory loss. Scientists have also found several gene mutations that cause the disease to be passed on from parent to child.
The key to understanding whether Alzheimer’s is hereditary or not lies in the genetics of the disease. Genes encoding four main proteins have been identified so far in association with AD, amyloid precursor protein (APP), presenilin 1 (PSEN1), presenilin 2 (PSEN2), and apolipoprotein E (APOE). The first three gene mutations have been linked to early-onset (familial) Alzheimer’s disease, whereas the fourth one is considered to be a major risk factor in the late-onset of the disease.
The scientists showed in the early 1980s that an AD patient’s brain is characterized by an extensive distribution of two types of abnormal structures: senile plaques and neurofibrillary tangles (NFTs) (3). This led them to propose two main hypotheses to describe AD pathogenesis: the amyloid hypothesis and the tau hypothesis.
Senile plaques typically consist of a core of amyloid fibrils composed of the amyloid β (Aβ) peptide and are surrounded by degenerating neurons. According to the amyloid hypothesis, the production of Aβ in the brain initiates a series of events causing the clinical syndrome of dementia. Meanwhile, the tau hypothesis states that the hyperphosphorylation of tau is the primary cause of neurodegeneration in AD. NFT’s are composed of bundles of phosphorylated tau proteins. When tau is hyperphosphorylated, it dissociates from microtubules and aggregates into NFTs contributing to neural damage (4).
Alzheimer’s: symptoms & disease stagesThe first symptom of AD that manifests itself is difficulty remembering newly learned information. As Alzheimer's advances, it leads to more severe symptoms such as memory and orientation loss, confusion, and impaired judgment and decision making. These symptoms are often accompanied by neuropsychiatric ones such as depression, anxiety, and hallucinations.
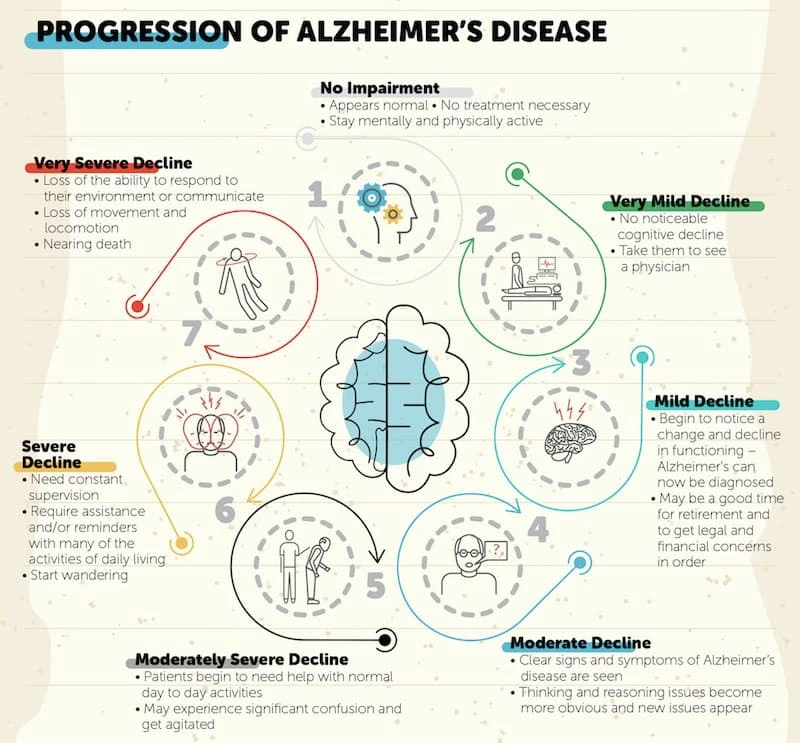
Alzheimer's disease typically progresses in stages and these can be presented in a simple three-stage breakdown as described below or in a more detailed seven-stage framework as shown in the figure below.
- Mild Alzheimer's (early stage)
- Moderate Alzheimer's (middle stage)
- Severe Alzheimer's (late stage)
The first symptom of AD that manifests itself is difficulty remembering newly learned information. As Alzheimer's advances, it leads to more severe symptoms such as memory and orientation loss, confusion, and impaired judgment and decision making. These symptoms are often accompanied by neuropsychiatric ones such as depression, anxiety, and hallucinations.
Alzheimer's disease typically progresses in stages and these can be presented in a simple three-stage breakdown as described below or in a more detailed seven-stage framework as shown in the figure below.
- Mild Alzheimer's (early stage)
- Moderate Alzheimer's (middle stage)
- Severe Alzheimer's (late stage)
Dementia vs. Alzheimer’s: what’s the difference?The main difference between dementia and Alzheimer’s disease is that dementia is a broader term representing a group of brain disorders that impact cognitive functions such as memory, communication, and reasoning while Alzheimer’s is a specific form of dementia. Alzheimer’s accounts for about 60% of all dementias and is the most prevalent subtype (2).
The main difference between dementia and Alzheimer’s disease is that dementia is a broader term representing a group of brain disorders that impact cognitive functions such as memory, communication, and reasoning while Alzheimer’s is a specific form of dementia. Alzheimer’s accounts for about 60% of all dementias and is the most prevalent subtype (2).
Alzheimer’s Research: Disease Treatments & Cure
Although a century has passed since Alzheimer’s was first discovered, only symptomatic treatments are currently available. Disease-modifying drug treatments capable of hindering the course of the disease are still unavailable. Hence, there has been a greater need for alternate strategies and recently, stem cell and gene therapies have cast a new hope for AD treatment.
Pharmacological therapy for Alzheimer’s diseaseEver since the beta-amyloid hypothesis was first postulated, scientists started extensively researching disease-modifying therapeutics for Alzheimer’s. However, all the approved drugs so far are only able to treat the symptoms. In the last decade, there have been over 400 failed clinical trials in the last 15 years.
A recent analysis showed that the success rate of pharmacological clinical trials involving Alzheimer’s between 2002 and 2012 was only 0.4%, indicating that a resounding 99.6% of them had failed (5).
Recently, Biogen’s drug aducanumab, which is an antibody reducing amyloid plaques in the brain, was approved by FDA. While this brings new promise in the Alzheimer’s treatment space, there is still a need to explore alternative therapies for this disease.
Ever since the beta-amyloid hypothesis was first postulated, scientists started extensively researching disease-modifying therapeutics for Alzheimer’s. However, all the approved drugs so far are only able to treat the symptoms. In the last decade, there have been over 400 failed clinical trials in the last 15 years.
A recent analysis showed that the success rate of pharmacological clinical trials involving Alzheimer’s between 2002 and 2012 was only 0.4%, indicating that a resounding 99.6% of them had failed (5).
Recently, Biogen’s drug aducanumab, which is an antibody reducing amyloid plaques in the brain, was approved by FDA. While this brings new promise in the Alzheimer’s treatment space, there is still a need to explore alternative therapies for this disease.
Stem cell therapy for Alzheimer’s diseaseThe immense potential of stem cells in tissue regeneration has led researchers to study their applications in the treatment of neurodegenerative diseases. They have the advantage of being able to integrate into existing neural networks and can also secrete an array of neurotrophic factors that control neuroplasticity and neurogenesis. Stem cells such as mesenchymal stem cells (MSCs), neural stem cells (NSCs), and induced pluripotent stem cells (iPSCs) have been extensively researched for the treatment of AD (6).
In particular, MSCs have received special interest, as they have shown a reduction of beta-amyloid (Aβ) levels & neuroinflammation, increased neurogenesis, and improved behavioral performance in AD-transgenic mice (7). In another study, transplantation of NSCs showed improved cognition in AD-mice (8). In spite of numerous studies in this area, cell replacement strategies are far from clinical application in AD. One of the main challenges is that prior to their clinical use, stem cells have to be pre-differentiated in vitro into different types of neuroblasts.
The immense potential of stem cells in tissue regeneration has led researchers to study their applications in the treatment of neurodegenerative diseases. They have the advantage of being able to integrate into existing neural networks and can also secrete an array of neurotrophic factors that control neuroplasticity and neurogenesis. Stem cells such as mesenchymal stem cells (MSCs), neural stem cells (NSCs), and induced pluripotent stem cells (iPSCs) have been extensively researched for the treatment of AD (6).
In particular, MSCs have received special interest, as they have shown a reduction of beta-amyloid (Aβ) levels & neuroinflammation, increased neurogenesis, and improved behavioral performance in AD-transgenic mice (7). In another study, transplantation of NSCs showed improved cognition in AD-mice (8). In spite of numerous studies in this area, cell replacement strategies are far from clinical application in AD. One of the main challenges is that prior to their clinical use, stem cells have to be pre-differentiated in vitro into different types of neuroblasts.
Gene therapy for Alzheimer’s diseaseThe “Alzheimer’s gene” or APOE is widely considered to be the leading genetic risk factor for the disease. Although it was first discovered way back in 1991, not many studies focused on it until recently, as the researchers did not have the right tools to manipulate genes. But with the recent advances in genetic engineering, the APOE gene is beginning to garner a lot of interest again.
While APOE4 is considered a risk factor for Alzheimer’s, APOE2 is the “protective” variant. In a recent study, scientists have shown in a mouse model that by administering a virus-vector mediated delivery of APOE2 to the brain, the negative influences of APOE4 can be negated (9). Another gene that has recently gained interest in AD treatment is PGC-1α. Scientists have shown that this gene might be able to improve mitochondrial dysfunction and prevent oxidative stress in AD (10).
The “Alzheimer’s gene” or APOE is widely considered to be the leading genetic risk factor for the disease. Although it was first discovered way back in 1991, not many studies focused on it until recently, as the researchers did not have the right tools to manipulate genes. But with the recent advances in genetic engineering, the APOE gene is beginning to garner a lot of interest again.
While APOE4 is considered a risk factor for Alzheimer’s, APOE2 is the “protective” variant. In a recent study, scientists have shown in a mouse model that by administering a virus-vector mediated delivery of APOE2 to the brain, the negative influences of APOE4 can be negated (9). Another gene that has recently gained interest in AD treatment is PGC-1α. Scientists have shown that this gene might be able to improve mitochondrial dysfunction and prevent oxidative stress in AD (10).
How CRISPR is Accelerating Alzheimer’s Research
With traditional AD drug treatments succeeded in offering only symptomatic relief and not being able to modify the disease, scientists have started looking at alternate strategies. After the breakthrough in gene editing using CRISPR-Cas9, the technology is gaining interest for its potential in manipulating the genes that affect the disease. Some of the recent studies using CRISPR for Alzheimer’s research have been briefly discussed below.
CRISPR enables large scale disease modeling studies for Alzheimer’sThere are about 50 genes associated with Alzheimer’s disease and related dementias (ADRD), the interplay between them is unclear. Recently, an initiative by the NIH, known as the Inducible Pluripotent Stem Cell Neurodegeneration Initiative (iNDI) aimed to address this issue. The goal of this project is to generate a standardized collection of isogenic iPSC lines carrying over 100 mutations associated with ADRD. iNDI utilized CRISPR editing to generate multiple SNVs per gene across several targets in induced pluripotent stem cells. Learn more about the iNDI project here.
There are about 50 genes associated with Alzheimer’s disease and related dementias (ADRD), the interplay between them is unclear. Recently, an initiative by the NIH, known as the Inducible Pluripotent Stem Cell Neurodegeneration Initiative (iNDI) aimed to address this issue. The goal of this project is to generate a standardized collection of isogenic iPSC lines carrying over 100 mutations associated with ADRD. iNDI utilized CRISPR editing to generate multiple SNVs per gene across several targets in induced pluripotent stem cells. Learn more about the iNDI project here.
CRISPR helps identify new proteins & biomarkers for Alzheimer’sResearchers from the University of Tokyo used a CRISPR-Cas9 screening technique to identify a protein, calcium, and integrin-binding protein 1 (CIB1), involved in causing Alzheimer’s.
Another team of scientists from Spain observed that STIM1 (Stromal Interaction Molecule 1- an endoplasmic reticulum protein) expression levels decreased in the medial frontal gyrus of AD patients with the progression of neurogenesis. They used CRISPR-Cas9 to disrupt the expression of STIM1 gene in the SH-SY5Y neuroblastoma cell line. Using this they were further able to demonstrate that STIM1 deficiency triggers cell death due to change in the transport of calcium ions through the plasma membrane of neurons.
Identification of this new biomarker will be useful in understanding the role of STIM1 in the pathogenesis of the disease (11).
Researchers from the University of Tokyo used a CRISPR-Cas9 screening technique to identify a protein, calcium, and integrin-binding protein 1 (CIB1), involved in causing Alzheimer’s.
Another team of scientists from Spain observed that STIM1 (Stromal Interaction Molecule 1- an endoplasmic reticulum protein) expression levels decreased in the medial frontal gyrus of AD patients with the progression of neurogenesis. They used CRISPR-Cas9 to disrupt the expression of STIM1 gene in the SH-SY5Y neuroblastoma cell line. Using this they were further able to demonstrate that STIM1 deficiency triggers cell death due to change in the transport of calcium ions through the plasma membrane of neurons.
Identification of this new biomarker will be useful in understanding the role of STIM1 in the pathogenesis of the disease (11).
CRISPR-editing is set to make Alzheimer’s prevention a real possibilityRecently, researchers from Laval University in Canada used CRISPR to edit genes in brain cells to prevent Alzheimer’s. The team led by Jacques Tremblay identified a genetic variant called A673T, which can reduce Alzheimer’s biomarker beta-amyloid and also been found to reduce Alzheimer’s likelihood by a factor of four. The researchers successfully used CRISPR in petri dish studies to activate the A673T variant in approximately 40 percent of the lab-grown brain cells.
While it is still too soon to get too excited about this possibility, the next step would be to replicate the findings in animal studies.
Recently, researchers from Laval University in Canada used CRISPR to edit genes in brain cells to prevent Alzheimer’s. The team led by Jacques Tremblay identified a genetic variant called A673T, which can reduce Alzheimer’s biomarker beta-amyloid and also been found to reduce Alzheimer’s likelihood by a factor of four. The researchers successfully used CRISPR in petri dish studies to activate the A673T variant in approximately 40 percent of the lab-grown brain cells.
While it is still too soon to get too excited about this possibility, the next step would be to replicate the findings in animal studies.
Using CRISPR to correct AD mutations in presenilin genesMutations in the presenilin 2 (PSEN2) gene have been associated with early-onset familial Alzheimer’s disease. In this study, CRISPR was used to correct a presenilin 2 mutation in iPSC-derived neurons.
One of the first cell types to be affected in AD is the basal forebrain cholinergic neurons (BFCNs). Their dysfunction has been associated with impaired short-term memory. The researchers generated human BFCNs from iPSCs, using cell lines from PSEN2 mutation carriers/controls. The carriers displayed an increase in Aβ42/40 in BFCNs and also demonstrated an electrophysiological deficit. When CRISPR-Cas9 was used to correct the PSEN2 point mutation, the scientists were able to demonstrate normalization of the cells’ electrophysiological function and Aβ secretion (12).
Mutations in the PSEN1 gene have been associated with inherited AD. Another study was able to use CRISPR to correct the PSEN1 mutations in iPSCs (13).
Mutations in the presenilin 2 (PSEN2) gene have been associated with early-onset familial Alzheimer’s disease. In this study, CRISPR was used to correct a presenilin 2 mutation in iPSC-derived neurons.
One of the first cell types to be affected in AD is the basal forebrain cholinergic neurons (BFCNs). Their dysfunction has been associated with impaired short-term memory. The researchers generated human BFCNs from iPSCs, using cell lines from PSEN2 mutation carriers/controls. The carriers displayed an increase in Aβ42/40 in BFCNs and also demonstrated an electrophysiological deficit. When CRISPR-Cas9 was used to correct the PSEN2 point mutation, the scientists were able to demonstrate normalization of the cells’ electrophysiological function and Aβ secretion (12).
Mutations in the PSEN1 gene have been associated with inherited AD. Another study was able to use CRISPR to correct the PSEN1 mutations in iPSCs (13).
CRISPR-edited APP gene offers treatment possibilitiesThe Swedish mutation (APPswe) in the amyloid precursor protein (APP) gene has been associated with dominantly inherited Alzheimer’s disease. In this study, scientists used CRISPR to specifically target and disrupt the mutant allele of this gene. They were also able to show both ex vivo and in vivo that as a result of this, there was a decreased pathogenic Aβ. The results of this study demonstrate that this could be a potential treatment strategy in the future (14).
Another team led by Dr. Takaomi Saido, has identified protective mutations in the 3’ UTR of the APP gene in mice. They then used CRISPR to replace the wild-type APP gene with the mutated versions to induce a rodent version of Alzheimer’s disease. The results of their research showed that the mice with mutated forms of the APP gene were protected against cognitive decline and displayed lower levels of accumulated amyloid plaques.
The Swedish mutation (APPswe) in the amyloid precursor protein (APP) gene has been associated with dominantly inherited Alzheimer’s disease. In this study, scientists used CRISPR to specifically target and disrupt the mutant allele of this gene. They were also able to show both ex vivo and in vivo that as a result of this, there was a decreased pathogenic Aβ. The results of this study demonstrate that this could be a potential treatment strategy in the future (14).
Another team led by Dr. Takaomi Saido, has identified protective mutations in the 3’ UTR of the APP gene in mice. They then used CRISPR to replace the wild-type APP gene with the mutated versions to induce a rodent version of Alzheimer’s disease. The results of their research showed that the mice with mutated forms of the APP gene were protected against cognitive decline and displayed lower levels of accumulated amyloid plaques.
Future of CRISPR in Alzheimer’s Research
The application of CRISPR-Cas9 in Alzheimer’s research is still in its infancy. Although it might be too soon for CRISPR to find a use as a therapeutic tool, it can certainly play a crucial role in enabling scientists to study the pathogenesis of AD. To find a treatment for the disease, it is important to understand the underlying disruption in processes that contribute to it. CRISPR is the perfect tool for gene disruption and can assist scientists in identifying the role a particular gene might play in the progression of the disease.
One of the hurdles CRISPR faces before it can be ready to be used as a therapeutic tool is off-target effects. However, clinical trials have shown that CRISPR-based therapy has already been successful in treating certain types of cancer and some blood diseases. It is only a matter of time before CRISPR trials get approved for brain disorders.
References
- James BD, Leurgans SE, Hebert LE, Scherr PA, Yaffe K, Bennett DA, Contribution of Alzheimer disease to mortality in the United States, Neurology. 2014 Mar 25; 82(12):1045-50.
- Yiannopoulou KG, Papageorgiou SG. Current and future treatments for Alzheimer's disease. Ther Adv Neurol Disord. 2013;6(1):19–33. doi:10.1177/1756285612461679
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. (1985). Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. U.S.A. 82, 4245–4249. 10.1073/pnas.82.12.4245
- Kametani F, Hasegawa M. Reconsideration of Amyloid Hypothesis and Tau Hypothesis in Alzheimer's Disease. Front Neurosci. 2018;12:25. Published 2018 Jan 30. doi:10.3389/fnins.2018.00025
- Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimers Res Ther. 2014;6:37.
- Kyeong-Ah Kwak, Seung-Pyo Lee, Jin-Young Yang, and Young-Seok Park, “Current Perspectives regarding Stem Cell-Based Therapy for Alzheimer’s Disease,” Stem Cells International, vol. 2018, Article ID 6392986, 14 pages, 2018
- Lee J, Kwon SJ, Kim JH, et al. Cerebrospinal fluid from Alzheimer's disease patients as an optimal formulation for therapeutic application of mesenchymal stem cells in Alzheimer's disease. Sci Rep. 2019;9(1):564. Published 2019 Jan 24. doi:10.1038/s41598-018-37252-9
- McGinley LM, Kashlan ON, Bruno ES, et al. Human neural stem cell transplantation improves cognition in a murine model of Alzheimer's disease. Sci Rep. 2018;8(1):14776. Published 2018 Oct 3. doi:10.1038/s41598-018-33017-6
- Zhao L, et al. Intracerebral adeno-associated virus gene delivery of apolipoprotein E2 markedly reduces brain amyloid pathology in Alzheimer’s disease mouse models. Neurobiol Aging. 2016;44:159–172.
- Sweeney G, Song J. The association between PGC-1α and Alzheimer's disease [published correction appears in Anat Cell Biol. 2016 Jun;49(2):163]. Anat Cell Biol. 2016;49(1):1–6. doi:10.5115/acb.2016.49.1.1
- Pascual-Caro C, Berrocal M, Lopez-Guerrero AM, Alvarez-Barrientos A, Pozo-Guisado E, Gutierrez-Merino C, Mata AM, Martin-Romero FJ (2018) STIM1 deficiency is linked to Alzheimer's disease and triggers cell death in SH-SY5Y cells by upregulation of L-type voltage-operated Ca(2+) entry. J Mol Med (Berl) 96:1061–1079
- Ortiz-Virumbrales M, Moreno CL, Kruglikov I, et al. CRISPR/Cas9-Correctable mutation-related molecular and physiological phenotypes in iPSC-derived Alzheimer's PSEN2 N141I neurons. Acta Neuropathol Commun. 2017;5(1):77. Published 2017 Oct 27. doi:10.1186/s40478-017-0475-z
- Pires C, Schmid B, Petraeus C et al. Generation of a gene-corrected isogenic control cell line from an Alzheimer's disease patient iPSC line carrying a A79V mutation in PSEN1. Stem Cell Res. 17(2), 285–288 (2016)
- György B, Lööv C, Zaborowski MP, et al. CRISPR/Cas9 Mediated Disruption of the Swedish APP Allele as a Therapeutic Approach for Early-Onset Alzheimer's Disease. Mol Ther Nucleic Acids. ;11:429–440. doi:10.1016/j.omtn.2018.03.007
- James BD, Leurgans SE, Hebert LE, Scherr PA, Yaffe K, Bennett DA, Contribution of Alzheimer disease to mortality in the United States, Neurology. 2014 Mar 25; 82(12):1045-50.
- Yiannopoulou KG, Papageorgiou SG. Current and future treatments for Alzheimer's disease. Ther Adv Neurol Disord. 2013;6(1):19–33. doi:10.1177/1756285612461679
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. (1985). Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. U.S.A. 82, 4245–4249. 10.1073/pnas.82.12.4245
- Kametani F, Hasegawa M. Reconsideration of Amyloid Hypothesis and Tau Hypothesis in Alzheimer's Disease. Front Neurosci. 2018;12:25. Published 2018 Jan 30. doi:10.3389/fnins.2018.00025
- Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimers Res Ther. 2014;6:37.
- Kyeong-Ah Kwak, Seung-Pyo Lee, Jin-Young Yang, and Young-Seok Park, “Current Perspectives regarding Stem Cell-Based Therapy for Alzheimer’s Disease,” Stem Cells International, vol. 2018, Article ID 6392986, 14 pages, 2018
- Lee J, Kwon SJ, Kim JH, et al. Cerebrospinal fluid from Alzheimer's disease patients as an optimal formulation for therapeutic application of mesenchymal stem cells in Alzheimer's disease. Sci Rep. 2019;9(1):564. Published 2019 Jan 24. doi:10.1038/s41598-018-37252-9
- McGinley LM, Kashlan ON, Bruno ES, et al. Human neural stem cell transplantation improves cognition in a murine model of Alzheimer's disease. Sci Rep. 2018;8(1):14776. Published 2018 Oct 3. doi:10.1038/s41598-018-33017-6
- Zhao L, et al. Intracerebral adeno-associated virus gene delivery of apolipoprotein E2 markedly reduces brain amyloid pathology in Alzheimer’s disease mouse models. Neurobiol Aging. 2016;44:159–172.
- Sweeney G, Song J. The association between PGC-1α and Alzheimer's disease [published correction appears in Anat Cell Biol. 2016 Jun;49(2):163]. Anat Cell Biol. 2016;49(1):1–6. doi:10.5115/acb.2016.49.1.1
- Pascual-Caro C, Berrocal M, Lopez-Guerrero AM, Alvarez-Barrientos A, Pozo-Guisado E, Gutierrez-Merino C, Mata AM, Martin-Romero FJ (2018) STIM1 deficiency is linked to Alzheimer's disease and triggers cell death in SH-SY5Y cells by upregulation of L-type voltage-operated Ca(2+) entry. J Mol Med (Berl) 96:1061–1079
- Ortiz-Virumbrales M, Moreno CL, Kruglikov I, et al. CRISPR/Cas9-Correctable mutation-related molecular and physiological phenotypes in iPSC-derived Alzheimer's PSEN2 N141I neurons. Acta Neuropathol Commun. 2017;5(1):77. Published 2017 Oct 27. doi:10.1186/s40478-017-0475-z
- Pires C, Schmid B, Petraeus C et al. Generation of a gene-corrected isogenic control cell line from an Alzheimer's disease patient iPSC line carrying a A79V mutation in PSEN1. Stem Cell Res. 17(2), 285–288 (2016)
- György B, Lööv C, Zaborowski MP, et al. CRISPR/Cas9 Mediated Disruption of the Swedish APP Allele as a Therapeutic Approach for Early-Onset Alzheimer's Disease. Mol Ther Nucleic Acids. ;11:429–440. doi:10.1016/j.omtn.2018.03.007