In 2023, we entered the second decade of the CRISPR revolution, and saw the approval of Casgevy, the first CRISPR-based medicine to become widely available. The last several years have seen a marked increase in the number of genomic medicines in clinical development, and more of these candidates are pushing into the clinic for the first time. So how many CRISPR clinical trials are there? And where can you find the trial information?
We know it can be hard to keep up with all the developments in this rapid-paced field of research. But don’t worry, we’ve got you covered in this blog. We’ll explore a variety of current and upcoming CRISPR clinical trials, from genetic disorders to infectious diseases and cancer.
CRISPR Clinical Trials For Genetic Disorders
Genetic disorders are still one of the primary focus areas for the development of CRISPR-based medicines. There are currently many ongoing CRISPR clinical trials to treat various genetic diseases, including blood disorders, diabetes, cardiovascular diseases, immune disorders, and other rare disorders. Let’s delve into the various CRISPR clinical trials for genetic diseases.
CRISPR type 1 diabetes clinical trialCTX211 (VCTX210A)
Approach: ex vivo cell therapy
Indication/s: Type 1 diabetes
Sponsor:CRISPR Therapeutics
Phase: I/II
Trial ID number:NCT05210530
Developed by CRISPR Therapeutics, CTX211/VCTX210A is an immune-evasive, stem cell-derived beta-cell replacement therapy. It involves a transplant of allogeneic pancreatic endoderm cells (PEC210A) to patients with type I diabetes so they can produce their own insulin, without the need for chronic immunosuppression.
CRISPR-Cas9 is used to edit the healthy donor cells to enhance their fitness and allow them to evade the host immune system before they are transplanted into patients using a removable delivery device. The phase I/II CRISPR clinical trial of safety and efficacy expects to test the therapy in up to 40 patients and is estimated to be completed in August 2025.
CTX211 (VCTX210A)
Approach: ex vivo cell therapy
Indication/s: Type 1 diabetes
Sponsor:CRISPR Therapeutics
Phase: I/II
Trial ID number:NCT05210530
Developed by CRISPR Therapeutics, CTX211/VCTX210A is an immune-evasive, stem cell-derived beta-cell replacement therapy. It involves a transplant of allogeneic pancreatic endoderm cells (PEC210A) to patients with type I diabetes so they can produce their own insulin, without the need for chronic immunosuppression.
CRISPR-Cas9 is used to edit the healthy donor cells to enhance their fitness and allow them to evade the host immune system before they are transplanted into patients using a removable delivery device. The phase I/II CRISPR clinical trial of safety and efficacy expects to test the therapy in up to 40 patients and is estimated to be completed in August 2025.
CRISPR clinical trial for Duchenne muscular dystrophyHG-302
Approach: in vivo gene therapy
Indication/s: Duchenne muscular dystrophy
Sponsor:HuidaGene Therapeutics
Phase: I
Trial ID number: NCT06594094
HuidaGene Therapeutics announced in 2023 that their gene therapy for Duchenne Muscular Dystrophy (DMD) had been granted orphan drug designation by the FDA, in addition to its prior rare pediatric disease designation. The HG-302 therapy uses HuidaGene’s new engineered Cas12 nuclease, high-fidelity Cas12Max (hfCas12Max), to induce exon skipping in the splice-donor site at exon 51 of the DMD gene and restore the correct open reading frame.
Designed to rescue dystrophin expression and improve muscle function, HG-302 has shown promising preclinical data in humanized mice and monkeys. The use of compact hfCas12Max allows this in vivo gene therapy to be packaged in a single AAV vector for delivery to muscle tissue. In December 2024, HuidaGene announced they had dosed the first patient with HG-302 in their MUSCLE clinical trial.
HG-302
Approach: in vivo gene therapy
Indication/s: Duchenne muscular dystrophy
Sponsor:HuidaGene Therapeutics
Phase: I
Trial ID number: NCT06594094
HuidaGene Therapeutics announced in 2023 that their gene therapy for Duchenne Muscular Dystrophy (DMD) had been granted orphan drug designation by the FDA, in addition to its prior rare pediatric disease designation. The HG-302 therapy uses HuidaGene’s new engineered Cas12 nuclease, high-fidelity Cas12Max (hfCas12Max), to induce exon skipping in the splice-donor site at exon 51 of the DMD gene and restore the correct open reading frame.
Designed to rescue dystrophin expression and improve muscle function, HG-302 has shown promising preclinical data in humanized mice and monkeys. The use of compact hfCas12Max allows this in vivo gene therapy to be packaged in a single AAV vector for delivery to muscle tissue. In December 2024, HuidaGene announced they had dosed the first patient with HG-302 in their MUSCLE clinical trial.
Alternatives to CRISPR-Cas9: Nucleases for Next-Gen Therapy
There are now many alternatives to CRISPR-Cas9. In this blog, we explore CRISPR alternative nucleases and how they are being used to create new therapies.
CRISPR clinical trials for cardiovascular diseasesVERVE-101
Approach: in vivo gene therapy
Indication/s: High-risk heterozygous familial hypercholesterolemia (HeFH), established atherosclerotic cardiovascular disease (ASCVD), and patients with uncontrolled low-density lipoprotein C (LDL-C) levels.
Sponsor:Verve Therapeutics
Phase: Ib (enrollment paused)
Trial ID number:NCT05398029
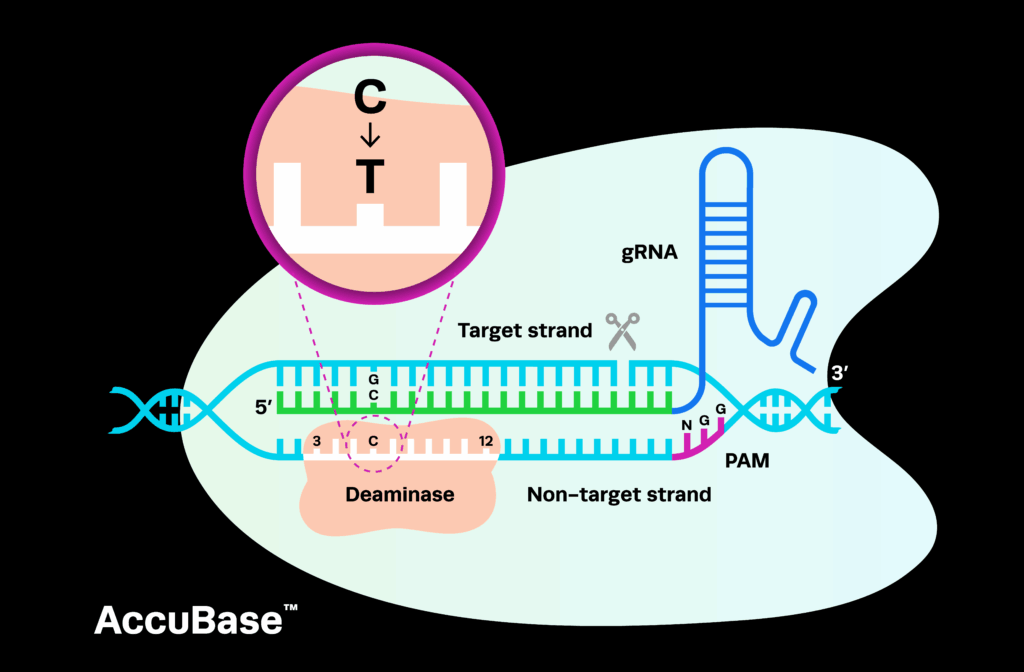
Sponsored by Verve Therapeutics, VERVE-101 uses an adenine base editing (ABE) system to inactivate the PCSK9 gene in vivo in the liver. This proof-of-concept therapy aims to lower the high levels of LDL-C in the blood of patients with HeFH, preventing plaque from building up in their arteries and reducing their risk of heart attack and stroke.
The heart-1 CRISPR clinical trial of VERVE-101 was the first base-editing approach to reach the clinic. However, after observing laboratory abnormalities associated with the treatment, Verve decided to pause enrollments and suspend the trial, instead focusing on VERVE-102.
VERVE-101
Approach: in vivo gene therapy
Indication/s: High-risk heterozygous familial hypercholesterolemia (HeFH), established atherosclerotic cardiovascular disease (ASCVD), and patients with uncontrolled low-density lipoprotein C (LDL-C) levels.
Sponsor:Verve Therapeutics
Phase: Ib (enrollment paused)
Trial ID number:NCT05398029
Sponsored by Verve Therapeutics, VERVE-101 uses an adenine base editing (ABE) system to inactivate the PCSK9 gene in vivo in the liver. This proof-of-concept therapy aims to lower the high levels of LDL-C in the blood of patients with HeFH, preventing plaque from building up in their arteries and reducing their risk of heart attack and stroke.
The heart-1 CRISPR clinical trial of VERVE-101 was the first base-editing approach to reach the clinic. However, after observing laboratory abnormalities associated with the treatment, Verve decided to pause enrollments and suspend the trial, instead focusing on VERVE-102.
VERVE-102
Approach: in vivo gene therapy
Indication/s: HeFH, coronary artery disease (CAD)
Sponsor: Verve Therapeutics
Phase: Ib
Trial ID number: NCT06164730
VERVE-102 is another approach by Verve Therapeutics to inactivate PCSK9 using base editors. This therapy is delivered with Verve’s proprietary GalNAc-lipid nanoparticle (LNP) technology, using a different delivery system to VERVE-101. The Heart-2 trial evaluating VERVE-102 is likely to include four dose cohorts of the therapy, with three to nine patients in each.
Preliminary results from late 2024 showed that the therapy has been well-tolerated by the first two dose cohorts, with no serious adverse events or laboratory anomalies. An update is expected in the first half of 2025.
VERVE-201
Approach: in vivo gene therapy
Indication/s: Refractory hyperlipidemia (RH), homozygous familial hypercholesterolemia (HoFH)
Sponsor:Verve Therapeutics
Phase: Ib
Trial ID number:NCT06451770
VERVE-201 was designed to permanently switch off the ANGPTL3 gene in the liver in order to lower LDL-C levels and remnant cholesterol. Utilizing Verve’s GalNAc-LNP delivery technology, VERVE-201 is currently being evaluated in the Pulse-1 clinical trial, in patients with RH. The first patient was dosed in November 2024.
CTX310
Approach: in vivo gene therapy
Indication/s: Homozygous or heterozygous familial hypercholesterolemia, severe hypertriglyceridemia, mixed dyslipidemias
Sponsor: CRISPR Therapeutics
Phase: I
CRISPR Therapeutics developed CTX310 to knock out the angiopoietin-like 3 protein (ANGPTL3) gene, a key regulator of lipid metabolism. The therapy aims to reduce levels of serum lipids and lower the risk of ASVCD in patients.
CTX310 uses CRISPR-Cas9 for editing and is delivered to the liver via LNP. Preclinical studies in non-human primates (NHPs) demonstrated durable reductions in the ANGPTL3 protein and triglycerides after a single treatment. A phase I CRISPR clinical trial has been initiated for CTX310, and updates are expected in the first half of 2025.
CTX320
Approach: in vivo gene therapy
Indication/s: Cardiovascular disease (high Lp(a))
Sponsor: CRISPR Therapeutics
Phase: Preclinical
CTX320 is designed to reduce the expression of lipoprotein a (Lp(a)), elevated levels of which are a strong risk factor for atherosclerosis and related diseases. As with CTX310, Cas9 and a guide RNA targeting the gene are delivered to the liver via LNP. Preclinical research in NHPs demonstrated a durable reduction in Lp(a) levels after a single dose of CTX320. A CRISPR clinical trial for the therapy began in 2024 and an update should be coming by mid 2025.
ART002
Approach: in vivo gene therapy
Indication/s: Familial hypercholesterolemia
Sponsor:AccurEdit Therapeutics
AccurEdit Therapeutics developed their ART002 candidate to treat familial hypercholesterolemia by targeting the PCSK9 gene. Similar to CRISPR Therapeutics’ candidates, ART002 uses Cas9 and a single guide RNA, delivered in an LNP. A trial began in mid-2024 and is ongoing; a press release from AccurEdit in February 2025 asserted that the therapy has an excellent safety profile.
CRISPR chronic granulomatous disease clinical trialPM359
Approach: ex vivo cell therapy
Indication/s: Chronic granulomatous disease (CGD)
Sponsor:Prime Medicine
Phase: Preclinical (IND-enabling)
GCD is a rare genetic immune disorder that results in recurrent and potentially fatal infections. One of the causes of GCD is mutations in the NCF1 gene, which codes for an enzyme called NADPH oxidase.
Prime Medicine’s first clinical candidate, PM359 uses prime editors to correct mutations in the NCF1 gene ex vivo in patient CD34+ hematopoietic stem cells (HSCs). The FDA cleared Prime’s investigational new drug (IND) application for PM359 in 2024, and a phase I trial is predicted to begin in early 2025.
PM359
Approach: ex vivo cell therapy
Indication/s: Chronic granulomatous disease (CGD)
Sponsor:Prime Medicine
Phase: Preclinical (IND-enabling)
GCD is a rare genetic immune disorder that results in recurrent and potentially fatal infections. One of the causes of GCD is mutations in the NCF1 gene, which codes for an enzyme called NADPH oxidase.
Prime Medicine’s first clinical candidate, PM359 uses prime editors to correct mutations in the NCF1 gene ex vivo in patient CD34+ hematopoietic stem cells (HSCs). The FDA cleared Prime’s investigational new drug (IND) application for PM359 in 2024, and a phase I trial is predicted to begin in early 2025.
CRISPR clinical trial for transthyretin amyloidosisNTLA-2001 (nexiguran ziclumeran)
Approach: in vivo gene therapy
Indication/s: Transthyretin (ATTR) amyloidosis, ATTR amyloidosis with cardiomyopathy (ATTR-CM), hereditary ATTR with polyneuropathy (ATTRv-PN)
Sponsor:Intellia Therapeutics
Phase: III
Trial ID number:NCT06128629
A progressive and fatal disease, hereditary ATTR (ATTRv) is caused by mutations in the TTR gene, which results in the accumulation of misfolded transthyretin (TTR) protein. The buildup of these amyloid deposits causes damage to the peripheral nervous system and multiple organs. ATTRv can develop into polyneuropathy (ATTRv-PN) and cardiomyopathy (ATTR-CM). Interestingly, even individuals without mutations in TTR can produce normal TTR that misfolds and causes disease, a condition known as wild-type ATTR (ATTRwt).
Sponsored by Intellia Therapeutics and in collaboration with Regeneron, NTLA-2001, otherwise known as nexiguran ziclumeran or simply nex-z, aims to reduce levels of the circulating TTR protein in patients with ATTRv-CM and ATTRv-PN via CRISPR-Cas9 knockout of TTR. The MAGNITUDE phase III clinical trial is ongoing, assessing the safety and efficacy of a single dose of NTLA-2001 compared to a placebo in more than 700 patients.
NTLA-2001 (nexiguran ziclumeran)
Approach: in vivo gene therapy
Indication/s: Transthyretin (ATTR) amyloidosis, ATTR amyloidosis with cardiomyopathy (ATTR-CM), hereditary ATTR with polyneuropathy (ATTRv-PN)
Sponsor:Intellia Therapeutics
Phase: III
Trial ID number:NCT06128629
A progressive and fatal disease, hereditary ATTR (ATTRv) is caused by mutations in the TTR gene, which results in the accumulation of misfolded transthyretin (TTR) protein. The buildup of these amyloid deposits causes damage to the peripheral nervous system and multiple organs. ATTRv can develop into polyneuropathy (ATTRv-PN) and cardiomyopathy (ATTR-CM). Interestingly, even individuals without mutations in TTR can produce normal TTR that misfolds and causes disease, a condition known as wild-type ATTR (ATTRwt).
Sponsored by Intellia Therapeutics and in collaboration with Regeneron, NTLA-2001, otherwise known as nexiguran ziclumeran or simply nex-z, aims to reduce levels of the circulating TTR protein in patients with ATTRv-CM and ATTRv-PN via CRISPR-Cas9 knockout of TTR. The MAGNITUDE phase III clinical trial is ongoing, assessing the safety and efficacy of a single dose of NTLA-2001 compared to a placebo in more than 700 patients.
CRISPR hereditary angioedema clinical trialNTLA-2002
Type of therapy: in vivo gene therapy
Indication/s: Hereditary angioedema (HAE)
Sponsor: Intellia Therapeutics
Phase: I/II
Trial ID number:NCT05120830
HAE is a debilitating disease characterized by recurrent episodes of severe and potentially fatal swelling, including in the airways, face, and extremities. NTLA-2002 uses CRISPR-Cas9 delivered via LNP to disable the kallikrein B1 (KLKB1) gene in vivo. KLKB1 encodes a precursor of plasma kallikrein, which is involved in multiple inflammatory pathways that contribute to the disease.
NTLA-2002 is currently in phase I/II clinical trials for safety and tolerability, with an estimated enrollment of 55 adult patients. Early data from this trial demonstrates that it is well-tolerated by patients, and durable reductions in plasma kallikrein levels can be observed after a single dose, with a subsequent reduction in the number of swelling attacks experienced by the patients.
NTLA-2002
Type of therapy: in vivo gene therapy
Indication/s: Hereditary angioedema (HAE)
Sponsor: Intellia Therapeutics
Phase: I/II
Trial ID number:NCT05120830
HAE is a debilitating disease characterized by recurrent episodes of severe and potentially fatal swelling, including in the airways, face, and extremities. NTLA-2002 uses CRISPR-Cas9 delivered via LNP to disable the kallikrein B1 (KLKB1) gene in vivo. KLKB1 encodes a precursor of plasma kallikrein, which is involved in multiple inflammatory pathways that contribute to the disease.
NTLA-2002 is currently in phase I/II clinical trials for safety and tolerability, with an estimated enrollment of 55 adult patients. Early data from this trial demonstrates that it is well-tolerated by patients, and durable reductions in plasma kallikrein levels can be observed after a single dose, with a subsequent reduction in the number of swelling attacks experienced by the patients.
CRISPR in the Clinic: Synthego’s Regulatory Experts Discuss Development of Cell and Gene Therapies
The CRISPR gene therapy regulatory space is changing rapidly. In this blog, we’re shining a light on regulatory scientists, the unsung heroes of clinical development.

CRISPR clinical trial for OTC deficiencyECUR-506/iECURE-OTC
Type of therapy: in vivo gene editing
Indication/s: Ornithine transcarbamylase (OTC) deficiency
Sponsor:Precision Biosciences
Phase: I/II
OTC deficiency is a rare X-linked urea cycle disorder characterized by a lack of the OTC enzyme that would normally convert ammonia to urea in the liver. OTC deficiency causes the buildup of ammonia in the blood, resulting in toxicity and potential brain damage.
Precision Biosciences employed their proprietary ARCUS platform to develop ECUR-506, also known as iECURE-OTC, to treat the disorder. Using a PCSK9-specific ARCUS meganuclease, ECUR-506 is designed to knock-in a functional copy of the OTC gene in the liver. A phase I/II clinical trial in infant boys is ongoing; preliminary data show that the therapy is well-tolerated and results in complete clinical response.
ECUR-506/iECURE-OTC
Type of therapy: in vivo gene editing
Indication/s: Ornithine transcarbamylase (OTC) deficiency
Sponsor:Precision Biosciences
Phase: I/II
OTC deficiency is a rare X-linked urea cycle disorder characterized by a lack of the OTC enzyme that would normally convert ammonia to urea in the liver. OTC deficiency causes the buildup of ammonia in the blood, resulting in toxicity and potential brain damage.
Precision Biosciences employed their proprietary ARCUS platform to develop ECUR-506, also known as iECURE-OTC, to treat the disorder. Using a PCSK9-specific ARCUS meganuclease, ECUR-506 is designed to knock-in a functional copy of the OTC gene in the liver. A phase I/II clinical trial in infant boys is ongoing; preliminary data show that the therapy is well-tolerated and results in complete clinical response.
CRISPR Alpha-1 antitrypsin deficiency therapyBEAM-302
Type of therapy: in vivo gene editing
Indication/s: Alpha-1 antitrypsin deficiency (AATD)
Sponsor: Beam Therapeutics
Phase: I/II
Trial ID number:NCT06389877
Beam’s BEAM-302 therapy is designed to treat AATD, in which a lack of AAT protein results in lung and liver damage. The most common cause of AATD is the homozygous PiZ variant in the SERPINA1 gene. Using base editors delivered via a liver-targeted LNP, BEAM-302 aims to correct the PiZ variant.
Beam announced positive initial data from their phase I/II clinical trial in March 2025, reporting that the therapy is well-tolerated and that a single dose of BEAM-302 led to durable increases in functional AAT. The trial is ongoing and is currently recruiting.
BEAM-302
Type of therapy: in vivo gene editing
Indication/s: Alpha-1 antitrypsin deficiency (AATD)
Sponsor: Beam Therapeutics
Phase: I/II
Trial ID number:NCT06389877
Beam’s BEAM-302 therapy is designed to treat AATD, in which a lack of AAT protein results in lung and liver damage. The most common cause of AATD is the homozygous PiZ variant in the SERPINA1 gene. Using base editors delivered via a liver-targeted LNP, BEAM-302 aims to correct the PiZ variant.
Beam announced positive initial data from their phase I/II clinical trial in March 2025, reporting that the therapy is well-tolerated and that a single dose of BEAM-302 led to durable increases in functional AAT. The trial is ongoing and is currently recruiting.
CRISPR Clinical Trials for Infectious Diseases
There are now several different CRISPR clinical trials tackling infectious diseases, from CRISPR-enhanced bacteriophages to the excision of integrated retroviruses, and even epigenetic silencing of entire viral genomes. In this section, we’ll take a look at the leading candidates in CRISPR clinical trials for infectious diseases.
CRISPR HIV Clinical TrialEBT-101
Type of therapy: in vivo CRISPR therapy
Indication/s: Human immunodeficiency virus (HIV)
Sponsor:Excision Biotherapeutics
Phase: I/II (completed)
Trial ID number:NCT05144386
HIV is an integrating retrovirus that destroys CD4+ T cells, compromising the adaptive immune system. Developed by Excision Biotherapeutics, EBT-101 aims to cut the virus from the genome of human cells using CRISPR-Cas9 and two guide RNAs, delivered via AAV9. A phase I/II trial of the therapy was completed in 2024. While the therapy had a positive safety profile, it was unable to prevent viral rebound in patients who had stopped taking standard anti-retroviral therapies.
EBT-101
Type of therapy: in vivo CRISPR therapy
Indication/s: Human immunodeficiency virus (HIV)
Sponsor:Excision Biotherapeutics
Phase: I/II (completed)
Trial ID number:NCT05144386
HIV is an integrating retrovirus that destroys CD4+ T cells, compromising the adaptive immune system. Developed by Excision Biotherapeutics, EBT-101 aims to cut the virus from the genome of human cells using CRISPR-Cas9 and two guide RNAs, delivered via AAV9. A phase I/II trial of the therapy was completed in 2024. While the therapy had a positive safety profile, it was unable to prevent viral rebound in patients who had stopped taking standard anti-retroviral therapies.
CRISPR epigenetic editing tackles hepatitis BTUNE-401
Type of therapy: in vivo CRISPR therapy
Indication/s: Hepatitis B
Sponsor:Tune Therapeutics
Phase: Ib
Tune Therapeutics is taking a very different approach in the infectious disease space. Their ‘genetic tuning’ technology, ‘TEMPO’, uses CRISPR to modify the epigenome in vivo. Their leading clinical candidate TUNE-401 uses CRISPR-Cas9 epigenetic editing to treat hepatitis B virus (HBV), silencing all forms of the virus in the human liver by targeting a single, highly conserved sequence in its genome. Tune received FDA approval to begin a phase Ib clinical trial of TUNE-401 at the end of 2024.
TUNE-401
Type of therapy: in vivo CRISPR therapy
Indication/s: Hepatitis B
Sponsor:Tune Therapeutics
Phase: Ib
Tune Therapeutics is taking a very different approach in the infectious disease space. Their ‘genetic tuning’ technology, ‘TEMPO’, uses CRISPR to modify the epigenome in vivo. Their leading clinical candidate TUNE-401 uses CRISPR-Cas9 epigenetic editing to treat hepatitis B virus (HBV), silencing all forms of the virus in the human liver by targeting a single, highly conserved sequence in its genome. Tune received FDA approval to begin a phase Ib clinical trial of TUNE-401 at the end of 2024.
PBGENE-HBV
Type of therapy: in vivo CRISPR therapy
Indication/s: Hepatitis B
Sponsor:Precision Biosciences
Phase: I
Precision Biosciences are also taking aim at HBV. In March 2025, the company announced they had received FDA clearance of their IND application for PBGENE-HBV, a therapeutic candidate that uses their proprietary ARCUS meganuclease platform and is delivered via LNP. The ELIMINATE-B clinical trial has begun enrolling patients, and will evaluate multiple ascending doses of the therapy. Early results showed that the therapy is safe and well tolerated at the lowest dose.
CRISPR phage therapies for bacterial infectionsLBP-EC01
Type of therapy: in vivo CRISPR phage therapy
Indication/s: Urinary tract infections (UTIs) caused by multi-drug resistant E. coli
Sponsor:Locus Biosciences
Phase: II/III
Trial ID number:NCT05488340
CRISPR is being used by Locus Biosciences to create more effective phage therapies for bacterial infections. Locus’s LBP-EC01 therapy is an engineered bacteriophage ‘cocktail’, in which the phage have been endowed with the DNA-shredding CRISPR-Cas3 construct and guides targeting the E. coli genome.
The world-first ELIMINATE clinical trial, currently in phase II/III, is testing the therapy in patients with acute UTIs that are caused by multi-drug resistant E. coli. You can read the initial results in their 2024 publication in The Lancet. Locus also has several preclinical candidates targeting other bacterial species, including Staphylococcus aureus and Pseudomonas aeruginosa.
LBP-EC01
Type of therapy: in vivo CRISPR phage therapy
Indication/s: Urinary tract infections (UTIs) caused by multi-drug resistant E. coli
Sponsor:Locus Biosciences
Phase: II/III
Trial ID number:NCT05488340
CRISPR is being used by Locus Biosciences to create more effective phage therapies for bacterial infections. Locus’s LBP-EC01 therapy is an engineered bacteriophage ‘cocktail’, in which the phage have been endowed with the DNA-shredding CRISPR-Cas3 construct and guides targeting the E. coli genome.
The world-first ELIMINATE clinical trial, currently in phase II/III, is testing the therapy in patients with acute UTIs that are caused by multi-drug resistant E. coli. You can read the initial results in their 2024 publication in The Lancet. Locus also has several preclinical candidates targeting other bacterial species, including Staphylococcus aureus and Pseudomonas aeruginosa.
SNIPR001
Type of therapy: in vivo CRISPR phage therapy
Indication/s: E. coli
Sponsor:SNIPR Biome
Phase: I
Trial ID number:NCT05277350
Taking a similar approach to Locus Biosciences, SNIPR Biome has armed bacteriophage with CRISPR-Cas3 to selectively eradicate E. coli in the digestive tract and bloodstream. Their SNIPR001 therapy completed a phase I dose escalation trial in 36 participants in May 2023. The treatment was able to successfully reduce E. coli load in the digestive tract.
A new trial will evaluate SNIPR-001’s ability to clear fluoroquinolone-resistant E. coli infections from the bloodstream of hematological cancer patients who are undergoing hematopoietic stem cell transplantation.
CRISPR Cancer Clinical TrialsCRISPR-edited chimeric antigen receptor (CAR) T cell therapies are leading the fight against hematological malignancies in the clinic. However, some therapies are also being developed for solid tumors with the help of CRISPR technology. Let’s explore some of the key therapies in CRISPR cancer clinical trials.
CRISPR-edited chimeric antigen receptor (CAR) T cell therapies are leading the fight against hematological malignancies in the clinic. However, some therapies are also being developed for solid tumors with the help of CRISPR technology. Let’s explore some of the key therapies in CRISPR cancer clinical trials.
BEAM-201
Type of therapy: CAR-T cell therapy
Indication/s: Relapsed/
Sponsor:BEAM Therapeutics
Phase: I/II
Trial ID number:NCT05885464
BEAM-201 is an allogeneic, quadruple-edited anti-CD7 CAR-T cell therapy designed to treat patients with relapsed or refractory T-ALL or T-LL - highly aggressive blood cancers for which there are limited treatment options. Base editing is used to knock out four key genes in the donor-derived cells: CD7, TRAC, PDCD1, and CD52.
The edits reduce the risk of graft-versus-host disease (GvHD) and T cell exhaustion, as well as allowing the transplanted cells to survive in the presence of anti-CD52 agents and the host immune system. The phase I/II trial is ongoing, however, early efficacy data are encouraging.
CTX112
Approach: CAR-T cell therapy
Indication/s: Relapsed or refractory B-cell malignancies, autoimmune disease
Sponsor: CRISPR Therapeutics
Phase: I/II
Trial ID number:NCT05643742
CTX112 is an allogeneic anti-CD19 CAR T cell therapy for the treatment of relapsed and refractory B cell malignancies. CRISPR-Cas9 edits are used to increase potency and reduce exhaustion in the donor-derived cells.
With an estimated enrollment of 120 patients, the therapy is currently being tested for safety and efficacy in the treatment of B cell malignancies. Preliminary data show that CTX112 is well-tolerated and has the potential to provide clinical benefit. Interestingly, CTX112 is also a candidate for the treatment of autoimmune diseases, for which a CRISPR clinical trial is expected to begin in the first half of 2024.
CTX131
Approach: CAR-T cell therapy
Indication/s: hematologic malignancies, solid tumor
Sponsor: CRISPR Therapeutics
Phase: I/II
Trial ID number:NCT05795595
CRISPR Therapeutics’ CTX131 therapy aims to treat both hematologic and solid cancers. The allogeneic anti-CD70 CAR T cell therapy has multiple CRISPR-Cas9 edits designed to enhance the potency of the cells, as well as reduce T cell exhaustion. An ongoing phase I/II CRISPR clinical trial for relapsed and refractory solid tumors is assessing the therapy in an estimated 250 patients and it is also being tested in blood cancers. Updates are expected some time in 2025.
FT825/ONO-8250
Approach: CAR-T cell therapy
Indication/s: Human epidermal growth factor receptor 2 (HER2)-expressing solid tumors
Sponsor:Fate Therapeutics
Phase: I
Trial ID number: NCT06241456
In collaboration with Ono Pharmaceutical, Fate Therapeutics has developed FT825/ONO-8250, an iPSC-derived CAR T cell therapy targeting HER2-expressing solid tumors. The therapy contains seven edits, including insertion of the HER2 antigen-binding domain, a CXCR2 receptor, chimeric TGF𝛽, IL-7/IL-7 receptor fusion (IL-7RF), and a high-affinity, non-cleavable CD16a receptor, as well as deletion of CD38 and the TRAC locus.
Together, these features allow the therapy to overcome some of the difficulties in treating solid tumors, improving trafficking of the T cells to the site of the tumor, promoting T-cell stemness and persistence, enhancing function, avoiding GvHD, redirecting immunosuppressive factors within the tumor, and improving therapeutic outcomes in concert with monoclonal antibody (mAb) therapy.
In January 2024, Fate announced that a phase I trial has been initiated to investigate FT825/ONO-8250 both as a monotherapy and in combination with mAb therapy. In November of that year they presented initial data from the trial, demonstrating that FT825 has a favorable safety profile and no exhaustion was observed in the transplanted cells.
Fate also has several other CRISPR-edited clinical candidates, all of which are off-the-shelf cell therapies derived from iPSCs: FT819, a CAR T cell therapy for leukemias; FT576, a CAR NK cell therapy for myeloma; and FT522, a CAR NK cell therapy for relapsed and refractory B-cell lymphoma.
CB-012
Approach: CAR-T cell therapy
Indication/s: Relapsed and refractory acute myeloid leukemia (AML)
Sponsor:Caribou Biosciences
Phase: I
Trial ID number:NCT06128044
Another off-the-shelf cell therapy specialist, Caribou Biosciences is investigating CB-012, an allogeneic CAR T cell therapy for AML targeting the C-type lectin-like molecule-1 (CLL-1). Caribou use Cas12a to create five different edits in the cells: knockout of the TRAC locus, PD-1, and B2M, and knock-in of the anti-CLL-1 CAR and a B2M-HLA-E-peptide fusion protein. A phase I clinical trial for the therapy is currently underway, with an estimated enrollment of 70 adult patients.
CRISPR Revolution in Cell & Gene Therapy
CRISPR cell and gene therapies are revolutionizing medicine, offering potential cures for cancer, genetic disorders, and infectious diseases. In this eBook, we explore the promise of CRISPR therapies, the main challenges in clinical development, and potential solutions to these obstacles.

CISH-inactivated TILs
Approach: Tumor-infiltrating lymphocyte (TIL) therapy
Indication/s: Non-small cell lung cancer (NSCLC), gastrointestinal cancer
Sponsor:Intima Bioscience
Phase: I/II
Trial ID number:NCT05566223, NCT04426669
Intima Bioscience are targeting a novel immune checkpoint in tumor-infiltrating lymphocyte (TIL) cells to treat multiple solid tumors. CISH (cytokine-induced SH2 protein) is a key immune checkpoint protein that regulates T cell function and signaling. Using Cas9 to delete CISH results in increased anti-tumor activity of the TILs. Two ongoing clinical trials are assessing the edited cells in non-small cell lung cancer and gastrointestinal cancers.
KSQ-001EX
Approach: TIL therapy
Indication/s: Melanoma, NSCLC, head and neck squamous cell carcinoma (HNSCC)
Sponsor:KSQ Therapeutics
Phase: I/II
Trial ID number:NCT0623788
Like Intima Bioscience, KSQ Therapeutics are also developing an edited TIL cell therapy for solid tumors. Using CRISPR-Cas9, KSQ have inactivated the SOCS1 gene, which controls the anti-tumor function, engraftment, and persistence of TILs. The FDA cleared KSQ’s IND application and a phase I/II clinical trial began in 2024.
Later in 2024, KSQ announced that the FDA had also cleared their KSQ-004EX therapy to begin trials in solid tumors. KSQ-004EX is a more advanced version of KSQ-001EX; in addition to SOCS1, Regnase1 is also inactivated to promote anti-tumor function and persistence of the TILs.
BE CAR7
Approach: CAR-T cell therapy
Indication/s: T cell malignancies
Sponsor: Great Ormond Street Hospital for Children NHS Foundation Trust
Phase: I
Trial ID number:NCT05397184
Developed by Waseem Qasim at University College London’s Great Ormond Street Hospital, the BE CAR7 T cell therapy is designed to treat T cell malignancies in pediatric patients. The T cells are first endowed with a CAR that has specificity to CD7, a protein expressed in T cell acute lymphoblastic leukemia (T-ALL). The cells are then base edited to inactivate the CD52 and CD7 receptors and the β chain of the αβ T cell receptor. The trial is currently recruiting.
How Can Synthego Support CRISPR Clinical Trials?
At Synthego, we’re committed to ensuring that genomic medicines will be safe and accessible to all. We provide a spectrum of high-quality sgRNAs to support sponsors of these therapies from proof-of-concept studies through to CRISPR clinical trials, as well as SpCas9, hfCas12Max, and eSPOT-On nucleases. Our state-of-the-art GMP production facility was designed to produce pure, GMP-grade sgRNA at scale to accelerate clinical development timeframes for CRISPR cell and gene therapies.
Beyond supplying high-quality reagents for CRISPR clinical trials, Synthego is dedicated to providing expert regulatory support to our clients, easing the transition from the lab bench to the clinic. Our team of regulatory affairs specialists can assist in the preparation of clinical documentation, support you in your interactions with regulatory bodies, and avoid common obstacles in the clinical translation of CRISPR therapies.