Drosophila melanogaster is the annoying fruit fly that you often find hovering over your bowl of fruit. But from a research perspective, it is much more than just a bothersome fly––it is a model organism that has been used to investigate and understand the roots of many human diseases. In fact, this little fly has contributed to many award-winning discoveries, including at least five Nobel Prizes.
Continue reading on to find out why Drosophila has had so much success as a model organism, and how CRISPR and Drosophila are teaming up to make significant breakthroughs in human disease research. Specifically, we will cover the following topics:
Drosophila Melanogaster: What Makes it the Perfect Organism for Biological Research?
For over a century now, Drosophila melanogaster has been one of the most extensively studied model organisms for genetic research. Scientists have tried to understand its biology and relate it to human biological processes. So what makes it an ideal model organism? We have more in common with this tiny fly than one might think. Let us take a look at some of the things that make it a perfect model organism.
Drosophila life cycle

One of the biggest reasons for the success of the fruit fly as a model system is the convenience of handling; they are easy to raise and maintain. The life cycle of a fruit fly is only about 12 days, and each female lays between 750 and 1500 eggs in her lifetime. These flies breed quickly and prolifically––which means a lot of them can be raised at once. But convenience and cost are not the only reasons this organism is so popular for human genetic research.
Manageable size of Drosophila chromosomesJust like the humans, the chromosomes of Drosophila melanogaster also occur in pairs. Unlike humans who have 23 pairs of chromosomes, the fly has only four, making them very manageable. Of the four, one pair are the sex chromosomes and the remaining three are autosomes. The fruit fly also has giant salivary gland chromosomes known as Polytene chromosomes. Their large size makes them convenient to work within the lab.
Just like the humans, the chromosomes of Drosophila melanogaster also occur in pairs. Unlike humans who have 23 pairs of chromosomes, the fly has only four, making them very manageable. Of the four, one pair are the sex chromosomes and the remaining three are autosomes. The fruit fly also has giant salivary gland chromosomes known as Polytene chromosomes. Their large size makes them convenient to work within the lab.
Competitive Analysis of Guide RNA Formats
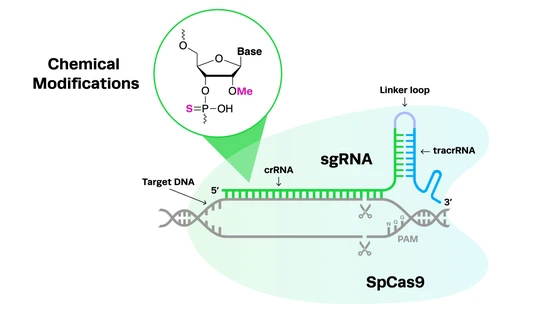
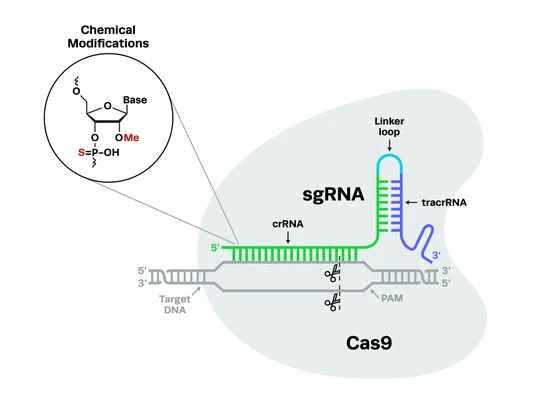
Guide RNAs come in two formats: two-piece cr:tracrRNA and single guide RNA (sgRNA), both with their advantages and disadvantages. At Synthego, we understand how important the right guide RNA format is for a successful CRISPR experiment. In this application note, we compare the editing efficiencies of Synthego’s cr:tracrRNA, cr:tracrRNA from other vendors, and Synthego’s sgRNA. Learn which is the best option for your experiment needs.

Drosophila genome: Do humans have more in common with the fruit fly than we think?The complete genome sequence of Drosophila was published in the year 2000. About a year later, studies comparing the fly sequence to that of the human genome sequence were published. The fly genome is only 5% of the size of the human genome, in terms of base pairs. However, the fly has about 15,500 genes distributed among its four chromosomes, while humans have close to 22,000 genes among their 23 chromosomes.
Humans and Drosophila have also retained the same genes from a common ancestor, which constitutes over 60% of their genome. Therefore, about 75% of genes associated with human genetic diseases and cancers have a counterpart in the fly genome. Understanding the fruit fly genetics and manipulating certain genes has allowed researchers to study the molecular mechanisms of pathological conditions such as cancer, neurological, cardiac, and metabolic diseases.
The complete genome sequence of Drosophila was published in the year 2000. About a year later, studies comparing the fly sequence to that of the human genome sequence were published. The fly genome is only 5% of the size of the human genome, in terms of base pairs. However, the fly has about 15,500 genes distributed among its four chromosomes, while humans have close to 22,000 genes among their 23 chromosomes.
Humans and Drosophila have also retained the same genes from a common ancestor, which constitutes over 60% of their genome. Therefore, about 75% of genes associated with human genetic diseases and cancers have a counterpart in the fly genome. Understanding the fruit fly genetics and manipulating certain genes has allowed researchers to study the molecular mechanisms of pathological conditions such as cancer, neurological, cardiac, and metabolic diseases.
Drosophila Models of Human Genetic Diseases and Biological Processes
As mentioned in the previous section, Drosophila has been used to model many human diseases. In this section, we will discuss some of the advances in human disease and biological processes research brought about by Drosophila.
The fly has been successfully used as a model organism to study cancer and has proven to be vital in describing several mechanisms fundamental to cancer development. Drosophila has been utilized to analyze the mechanisms underlying tumorigenesis and cancer progression.
The tiny fruit fly has also been successfully used to model many neurodegenerative diseases such as Huntington’s, Alzheimer’s and Parkinson’s diseases. Recently, a team of scientists identified many new mutations in genes that are associated with various aspects of the neural system like development, function, and maintenance.
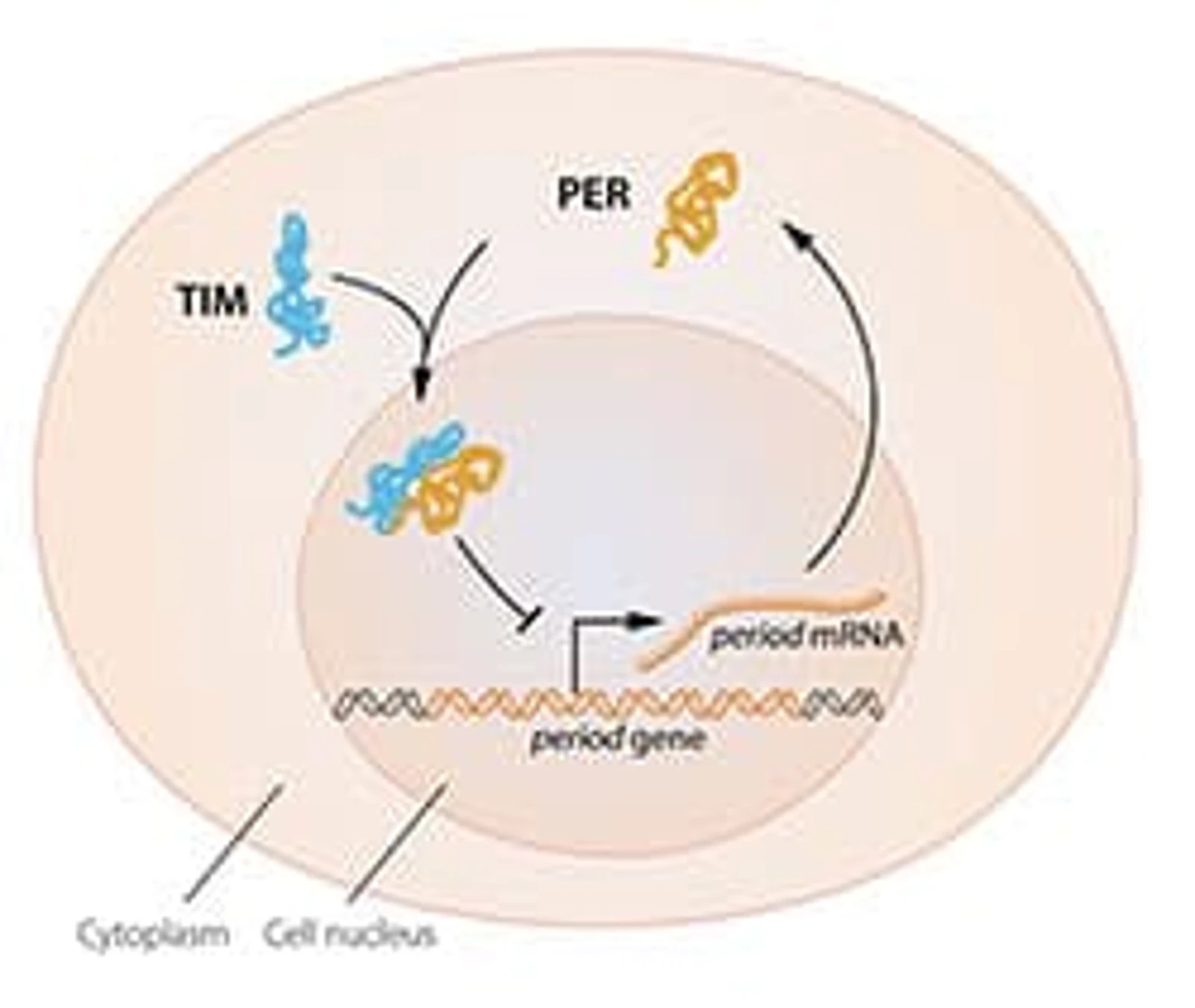
The most recent significant contribution by fruit fly to biological research has been the discoveries related to our sleep-awake cycles. By isolating the period gene and demonstrating that mutations of this gene led to the disruption of light-dark cycle in Drosophila, Jeffrey C. Hall, Michael Rosbash, and Michael W. Young, were able to explain how the clock system worked on a molecular level. This landmark discovery fetched them a Nobel Prize in 2017.
One of the reasons Drosophila is ideal for genetic research is that it is convenient and cost-effective to perform large-scale genetic screens of mutants relatively quickly. In Drosophila, RNAi has recently become a popular method to study the function of single genes or to probe gene function on a genome-wide scale. However, the RNAi technique can only reduce gene expression at the mRNA level instead of permanently silencing the gene. In contrast, the latest genome-editing technology––CRISPR can be utilized to introduce null mutations.
In the next section, we will discuss how CRISPR has the potential to advance genetic research in Drosophila.
CRISPR: Drosophila as a Model System for Advancing Genetic Research
The recent development of CRISPR technology is revolutionizing functional genomics research in many model organisms. This technology can be utilized to induce permanent mutations, and this is especially beneficial for loss-of-function analysis.
Large-scale CRISPR screening in DrosophilaTeams at German Cancer Research Center and the TRiP at Harvard Medical School are actively developing resources for large-scale CRISPR screening in Drosophila. Transgenic sgRNA libraries for loss-of-function and gain-of-function studies in vivo are being developed.
Suitability for genetic screens in Drosophila is a major reason for its popularity as a model organism. A team has recently achieved biallelic targeting of genes in selected somatic cells of the fly using CRISPR-Cas9. They demonstrated that it is feasible to restrict CRISPR-mediated mutagenesis and achieve high-throughput genetic screening in Drosophila heart.
Teams at German Cancer Research Center and the TRiP at Harvard Medical School are actively developing resources for large-scale CRISPR screening in Drosophila. Transgenic sgRNA libraries for loss-of-function and gain-of-function studies in vivo are being developed.
Suitability for genetic screens in Drosophila is a major reason for its popularity as a model organism. A team has recently achieved biallelic targeting of genes in selected somatic cells of the fly using CRISPR-Cas9. They demonstrated that it is feasible to restrict CRISPR-mediated mutagenesis and achieve high-throughput genetic screening in Drosophila heart.
In vivo CRISPR screening in Drosophila for neuronal modelingWhile CRISPR has been commonly used to generate germline mutations or perform in vitro screens, in vivo screening applications of CRISPR have been limited so far. Recently, a team of scientists characterized tissue-specific CRISPR (tsCRISPR) tools and optimized their use in a complex neuronal system of Drosophila mushroom body. They also demonstrated the application of the tsCRISPR approach in a large-scale in vivo screen to discover molecules required for developmental neuronal remodeling.
The applications of CRISPR technology in Drosophila that we have covered here offer only a glimpse into the innumerable ways researchers are likely to use it in the future. Together, CRISPR and Drosophila have the potential to answer many fundamental biological questions.
We hope you found this post useful. If you are interested in learning more about CRISPR, be sure to follow CRISPR in the news. You can also keep up with the latest CRISPR news by subscribing to our blog, or by following us on Twitter or Facebook!
While CRISPR has been commonly used to generate germline mutations or perform in vitro screens, in vivo screening applications of CRISPR have been limited so far. Recently, a team of scientists characterized tissue-specific CRISPR (tsCRISPR) tools and optimized their use in a complex neuronal system of Drosophila mushroom body. They also demonstrated the application of the tsCRISPR approach in a large-scale in vivo screen to discover molecules required for developmental neuronal remodeling.
The applications of CRISPR technology in Drosophila that we have covered here offer only a glimpse into the innumerable ways researchers are likely to use it in the future. Together, CRISPR and Drosophila have the potential to answer many fundamental biological questions.
We hope you found this post useful. If you are interested in learning more about CRISPR, be sure to follow CRISPR in the news. You can also keep up with the latest CRISPR news by subscribing to our blog, or by following us on Twitter or Facebook!
Superior Quality and Editing Performance of Synthetic sgRNAs
This application note demonstrates the superior quality and editing performance of Synthego’s chemically modified sgRNAs relative to modified sgRNAs from Vendor D.