Overcome Barriers of Gene Editing in iPS Cells
Learn how Synthego can deliver high-quality iPS cells with your desired edit, guaranteed.

Contents
Induced pluripotent stem (iPS) cells are one of the most valuable tools in biomedical research, and CRISPR gene editing technology has the potential to make them even more useful in many of their applications. However, there are significant challenges when attempting genetic manipulation in iPS cells which have hindered progress in the translation of these cells into clinical applications.
This in-depth CRISPR iPS cell blog will first explore the process of creating iPS cells, the advantages of iPS cells, the importance of CRISPR-Cas9 iPS cells, and some of the uses of iPS cell technology. Then we will discuss the various hurdles that come with CRISPR editing of iPS cells and how to overcome these.
What Are iPS Cells and Why Are They Important?
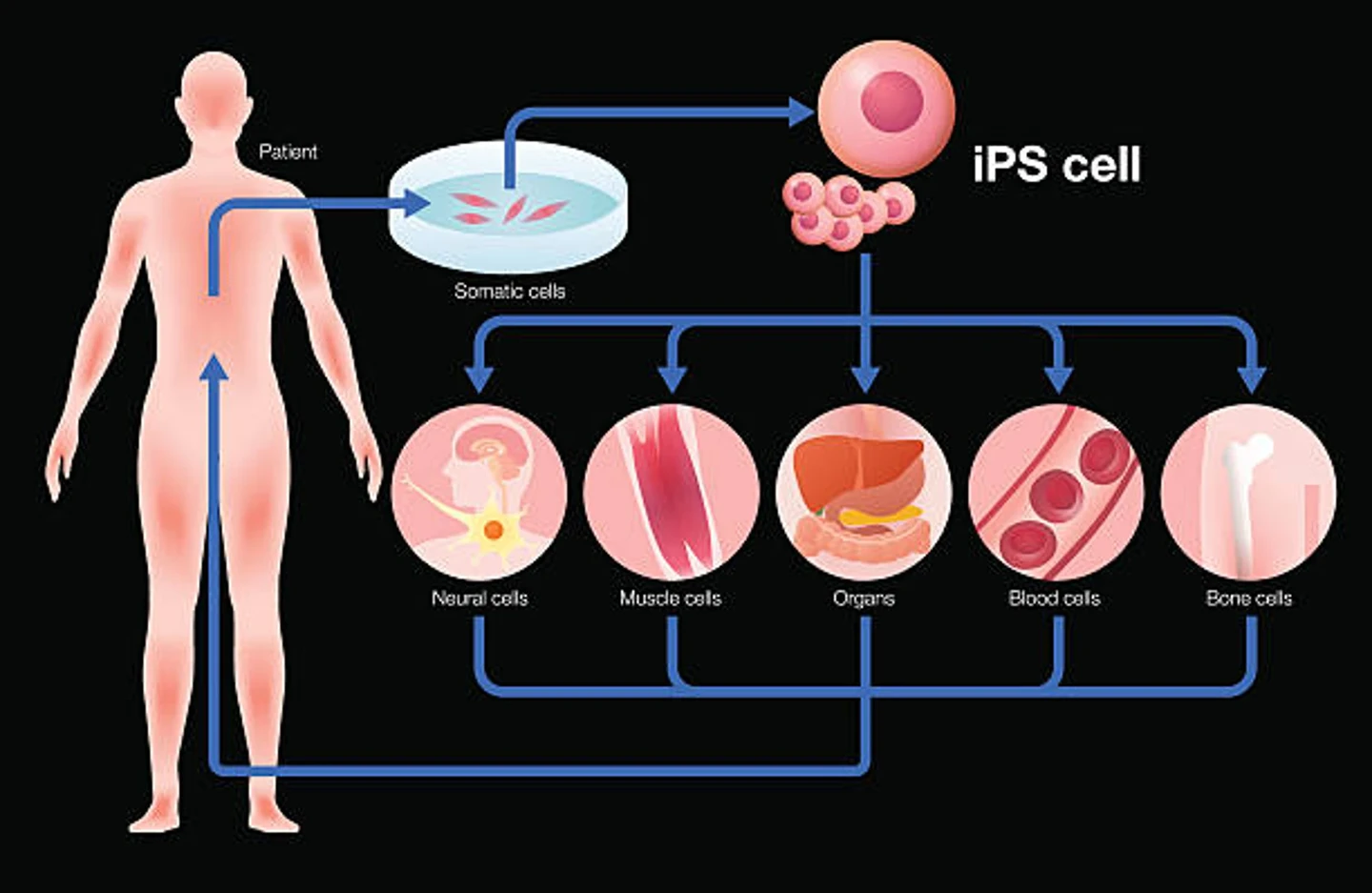
Unless you’re already familiar with them, you might be wondering, ‘what are induced pluripotent stem cells?’ So let’s start with the basics. Human induced pluripotent stem cells - known as iPS cells or iPSCs - are differentiated adult cells that have been reprogrammed back into a pluripotent state. First generated by Dr. Shinya Yamanaka in 2006, iPSCs represented a critical scientific breakthrough.
Because iPS cells are pluripotent, they can be used to generate virtually any cell type, making them incredibly valuable in many fields of research. Let’s explore how scientists go about creating iPS cells, how iPS cells differ from embryonic stem cells, and the advantages of using iPSCs.
Creating iPS cells: iPS cell reprogrammingCreating iPS cells starts with regular somatic cells, like skin or blood cells. The cells are treated with pluripotency-related transcription factors - Oct4, Klf4, Sox2, and c-Myc - so that they return to a pluripotent state, resembling embryonic stem cells. After iPS cell reprogramming, researchers can then propagate iPSCs indefinitely and use them to generate almost any other cell type of interest.
Creating iPS cells starts with regular somatic cells, like skin or blood cells. The cells are treated with pluripotency-related transcription factors - Oct4, Klf4, Sox2, and c-Myc - so that they return to a pluripotent state, resembling embryonic stem cells. After iPS cell reprogramming, researchers can then propagate iPSCs indefinitely and use them to generate almost any other cell type of interest.
iPS cells vs. embryonic stem cellsiPS cells are adult, differentiated skin or blood cells that have been reprogrammed back to pluripotency, while embryonic stem cells are naturally pluripotent cells harvested directly from real embryos. Harvesting of embryonic stem cells, and their use in research, brings with it a raft of moral and ethical concerns that have resulted in their use being prohibited in many parts of the world.
iPS cells are a key alternative to embryonic stem cells because they can be used to generate any type of cell but are harvested from adults in a non-invasive and ethical manner. Using iPSCs as a substitute for embryonic stem cells is becoming increasingly common in biomedical research.
iPS cells are adult, differentiated skin or blood cells that have been reprogrammed back to pluripotency, while embryonic stem cells are naturally pluripotent cells harvested directly from real embryos. Harvesting of embryonic stem cells, and their use in research, brings with it a raft of moral and ethical concerns that have resulted in their use being prohibited in many parts of the world.
iPS cells are a key alternative to embryonic stem cells because they can be used to generate any type of cell but are harvested from adults in a non-invasive and ethical manner. Using iPSCs as a substitute for embryonic stem cells is becoming increasingly common in biomedical research.
Advantages of iPS cells iPSCs can be used to generate virtually any cell type in the body, just like an embryonic stem cell. This versatility has made iPS cells invaluable to a variety of scientific fields, including functional genomics, disease modeling, drug discovery and development, cellular immunotherapies, and regenerative medicine.
As we’ve already mentioned, one of the advantages of iPS cells is that they can be generated from somatic cells sourced from patients and collected via relatively noninvasive procedures. In many respects, this eliminates the need to harvest stem cells from human embryos and cuts through a significant amount of ethical red tape.
iPSCs are also more reflective of biological complexity than many of the immortalized cell lines that have typically been used in research, making them a key alternative to these cell types. This is because iPSCs are collected directly from patients, enabling the generation of models and isogenic cell lines with patient-specific genetic backgrounds. With this unique capability, iPS cell technology is shaping the future of what is now known as personalized medicine.
iPSCs can be used to generate virtually any cell type in the body, just like an embryonic stem cell. This versatility has made iPS cells invaluable to a variety of scientific fields, including functional genomics, disease modeling, drug discovery and development, cellular immunotherapies, and regenerative medicine.
As we’ve already mentioned, one of the advantages of iPS cells is that they can be generated from somatic cells sourced from patients and collected via relatively noninvasive procedures. In many respects, this eliminates the need to harvest stem cells from human embryos and cuts through a significant amount of ethical red tape.
iPSCs are also more reflective of biological complexity than many of the immortalized cell lines that have typically been used in research, making them a key alternative to these cell types. This is because iPSCs are collected directly from patients, enabling the generation of models and isogenic cell lines with patient-specific genetic backgrounds. With this unique capability, iPS cell technology is shaping the future of what is now known as personalized medicine.
iPS Cell Technology and the Importance of Stem Cell Gene Editing
iPS cell technology is incredibly useful in its own right, however, when combined with CRISPR genome engineering, its range of capabilities is greatly expanded. In this section, we’ll discuss CRISPR iPS cells and the various applications of edited iPS cell technology.
CRISPR-Cas9 iPS cellsCompared to previous genome engineering technologies, CRISPR-Cas9 is much easier, cheaper, and more efficient, particularly when it comes to editing iPSCs. These advantages make it broadly applicable in induced pluripotent stem cell research. Firstly, CRISPR can be used in the reprogramming of iPS cells, eliminating the need to use viral vectors. It can also be used to introduce edits to the cells for the purpose of precision disease modeling or advanced cellular therapies (see the sections below).
CRISPR iPS cells are also incredibly valuable for drug discovery research, for several key reasons. Since the cells can be derived from patients, they are more clinically relevant for the study of particular diseases and they can also be differentiated into virtually any type of cell that requires study, reducing the need for harvesting primary cells or using immortalized cell lines. iPSC-derived cells can then be subjected to CRISPR genome-wide drug discovery screens, which help reveal the best therapeutic targets.
Genes in iPS cells to determine their function within certain cells or organs or their role in disease progression.
For example, CRISPR-edited iPSC neurons can be used as key models of neurological conditions. Dr. Lisa Ellerby at the Buck Institute uses iPSC neurons as model systems to mimic neurological diseases such as Huntington’s, Alzheimer’s, and Parkinson’s. Several studies have also demonstrated that disease-causing genetic mutations can be corrected in patient-derived CRISPR iPS cells, restoring normal cell function. These corrected cells can then be transplanted back into the patients from which they were derived, potentially curing the disease (see the iPS cell therapy section below).
A recent large-scale study using CRISPR iPS cells, the 2021 Inducible Pluripotent Stem Cell Neurodegeneration Initiative (iNDI), was performed as a collaboration between Synthego and the National Institutes of Health (NIH). This project used Synthego’s high-throughput ECLIPSE platform to generate 250 CRISPR iPS cell clones, making it the largest CRISPR-engineered iPSC study to date. These CRISPR iPSCs can be used as precision models of Alzheimer’s Disease and Related Dementias (ADRD), providing key avenues for further studies, including drug discovery and advanced disease modeling research.
Compared to previous genome engineering technologies, CRISPR-Cas9 is much easier, cheaper, and more efficient, particularly when it comes to editing iPSCs. These advantages make it broadly applicable in induced pluripotent stem cell research. Firstly, CRISPR can be used in the reprogramming of iPS cells, eliminating the need to use viral vectors. It can also be used to introduce edits to the cells for the purpose of precision disease modeling or advanced cellular therapies (see the sections below).
CRISPR iPS cells are also incredibly valuable for drug discovery research, for several key reasons. Since the cells can be derived from patients, they are more clinically relevant for the study of particular diseases and they can also be differentiated into virtually any type of cell that requires study, reducing the need for harvesting primary cells or using immortalized cell lines. iPSC-derived cells can then be subjected to CRISPR genome-wide drug discovery screens, which help reveal the best therapeutic targets.
Genes in iPS cells to determine their function within certain cells or organs or their role in disease progression.
For example, CRISPR-edited iPSC neurons can be used as key models of neurological conditions. Dr. Lisa Ellerby at the Buck Institute uses iPSC neurons as model systems to mimic neurological diseases such as Huntington’s, Alzheimer’s, and Parkinson’s. Several studies have also demonstrated that disease-causing genetic mutations can be corrected in patient-derived CRISPR iPS cells, restoring normal cell function. These corrected cells can then be transplanted back into the patients from which they were derived, potentially curing the disease (see the iPS cell therapy section below).
A recent large-scale study using CRISPR iPS cells, the 2021 Inducible Pluripotent Stem Cell Neurodegeneration Initiative (iNDI), was performed as a collaboration between Synthego and the National Institutes of Health (NIH). This project used Synthego’s high-throughput ECLIPSE platform to generate 250 CRISPR iPS cell clones, making it the largest CRISPR-engineered iPSC study to date. These CRISPR iPSCs can be used as precision models of Alzheimer’s Disease and Related Dementias (ADRD), providing key avenues for further studies, including drug discovery and advanced disease modeling research.
NIH Scientists Discuss the iNDI Project for Alzheimer’s Disease Modeling
The Inducible Pluripotent Stem Cell Neurodegeneration Initiative (iNDI), an NIH effort, aims to standardize disease models for Alzheimer’s and related dementias. Hear the principal contributors, Dr. Michael E. Ward and Dr. Mark R. Cookson, scientists at the NIH, talk about the inception of the iNDI project, the challenges and opportunities, and the future of neurodegenerative research.

CRISPR iPS cell therapyCRISPR iPS cells have enormous potential in the fields of cellular immunotherapy and regenerative medicine. For example, by using CRISPR to edit patient-derived iPSCs, disease-causing mutations can be corrected or replaced and the cells can be re-introduced back into patients as a type of ‘living drug’.
CRISPR iPS cells are also used to create cellular immunotherapies for the treatment of cancer. Previously, immunotherapies such as chimeric antigen receptor T cell therapy (CAR-T) required the harvesting of primary T cells from the blood of patients or healthy donors via leukapheresis before they could be edited and expanded into a therapeutic dose. This is problematic because patients undergoing cancer treatment often do not have immune cells to spare, and transplanted cells taken from healthy donors will be rejected by the host immune system or, conversely, may cause graft-versus-host disease (GvHD).
CRISPR iPS cells are a key alternative to this because they can be non-invasively harvested from skin cells. CRISPR knock-in of the relevant CAR allows the cells to detect and kill the patient’s tumor. If taken from healthy donors, iPSCs can be edited with CRISPR to generate ‘off-the-shelf’ universal T cell therapies, which have immune-recognition genes deleted so that they can be used to treat any patient.
Owing to their ability to differentiate into any type of cell, CRISPR iPS cells can also potentially be used to regenerate damaged tissues or organs. With CRISPR editing, patient-derived iPSCs could, for example, be used to regrow a liver for transplant with no fear of immune rejection and with any disease-causing mutations eliminated.
CRISPR iPS cells have enormous potential in the fields of cellular immunotherapy and regenerative medicine. For example, by using CRISPR to edit patient-derived iPSCs, disease-causing mutations can be corrected or replaced and the cells can be re-introduced back into patients as a type of ‘living drug’.
CRISPR iPS cells are also used to create cellular immunotherapies for the treatment of cancer. Previously, immunotherapies such as chimeric antigen receptor T cell therapy (CAR-T) required the harvesting of primary T cells from the blood of patients or healthy donors via leukapheresis before they could be edited and expanded into a therapeutic dose. This is problematic because patients undergoing cancer treatment often do not have immune cells to spare, and transplanted cells taken from healthy donors will be rejected by the host immune system or, conversely, may cause graft-versus-host disease (GvHD).
CRISPR iPS cells are a key alternative to this because they can be non-invasively harvested from skin cells. CRISPR knock-in of the relevant CAR allows the cells to detect and kill the patient’s tumor. If taken from healthy donors, iPSCs can be edited with CRISPR to generate ‘off-the-shelf’ universal T cell therapies, which have immune-recognition genes deleted so that they can be used to treat any patient.
Owing to their ability to differentiate into any type of cell, CRISPR iPS cells can also potentially be used to regenerate damaged tissues or organs. With CRISPR editing, patient-derived iPSCs could, for example, be used to regrow a liver for transplant with no fear of immune rejection and with any disease-causing mutations eliminated.
iPSC Gene editing: Challenges and Solutions
After hearing so much about the advantages of CRISPR iPS cells, you might be wondering what the catch is. And there certainly is one. So, what is the major challenge in CRISPR iPS cell generation? Put simply, genetically altering these cells while maintaining a high standard of quality is not easy. Difficulties often arise in keeping the cells alive, undifferentiated, and genetically stable.
Moreover, any CRISPR iPSC protocol will require high-quality reagents, optimized transfection procedures, and stringent quality control measures. If clones are needed, then the workflow includes extra hands-on time and constant monitoring over weeks. Any of these challenges can be a significant barrier to capitalizing on the benefits of CRISPR iPS cells. In this section, we’ll explore several of the key issues that researchers can encounter during iPS cell culture and the obstacles to editing these fickle experimental subjects.
iPSC differentiation: keeping iPS cells pluripotent is tricky
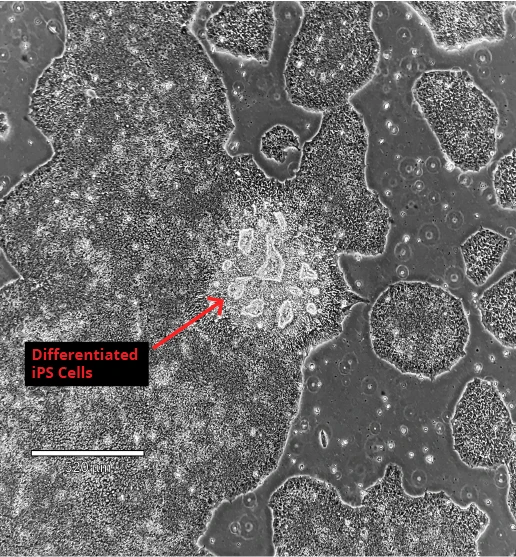
One of the intrinsic factors which makes iPS cells tricky to work with is that they require constant attention and maintenance - iPSC differentiation will always be an issue. Maintenance procedures include visualizing the cells on a daily basis in order to remove any differentiated cells and maintaining log phase growth. If this is neglected, iPSC differentiation will continue in your cell culture, leading to reduced pluripotency and other associated problems.
Solution: To ensure your iPS cell culture remains pluripotent, be sure to image the cells daily, remove any differentiated areas of the culture, passage (split) them during the log phase, and keep culture media fresh. For detailed protocols on freezing, thawing, maintaining, and passaging iPSCs, including troubleshooting tips, you can read this in-depth iPSC culturing guide from Sigma Aldrich.
iPS cells don’t like to be editedBefore the iPSC era, researchers used mouse embryonic stem cells to prove that genome editing in these cell types was possible. Technologies like zinc finger nucleases, TALENs, and CRISPR, all worked reasonably well in mouse embryonic stem cells. It seemed that these technologies would be easily translated into iPSCs when they became available.
To the surprise of many scientists, however, using these techniques to edit iPSCs proved much more difficult. Put simply, iPS cells don’t like to be genetically manipulated. Early iPS cell editing experiments were often inefficient or unsuccessful, not to mention extremely time-consuming.
One of the factors contributing to the resistance of iPSCs to genetic manipulation is that homology-directed repair (HDR) occurs even less frequently in iPS cells compared to immortal cell lines. Attempts to knock-in genes or correct mutations in iPS cells were largely unsuccessful because HDR was not occurring at high enough rates to repair the double-stranded breaks caused by CRISPR-Cas9. Performing multiple edits in iPSCs, which is often necessary to create models of multifactorial inheritance disorders, is even more challenging.
Solution: Since HDR only occurs at brief stages in the life cycle of cells, editing and reprogramming methods can be optimized by synchronizing the cell cycle in populations of iPSCs. This is usually achieved through methods like serum starvation when reprogramming iPSCs. However, a 2019 study screened small molecule drugs to identify a target that could synchronize iPSCs without affecting their unique characteristics.
Fortunately, it is much easier to edit iPSCs using CRISPR-Cas9 technology rather than earlier genome engineering technologies like TALENs and ZFNs. A key method to increase the likelihood of HDR for generating CRISPR iPS cells is by inhibiting the non-homologous end-joining (NHEJ) pathway, which is the more common DNA repair mechanism. A 2018 study found that by combining several different small molecule NHEJ inhibitors, they could achieve up to a 7.2 increase in CRISPR editing efficiencies in iPSCs.
Before the iPSC era, researchers used mouse embryonic stem cells to prove that genome editing in these cell types was possible. Technologies like zinc finger nucleases, TALENs, and CRISPR, all worked reasonably well in mouse embryonic stem cells. It seemed that these technologies would be easily translated into iPSCs when they became available.
To the surprise of many scientists, however, using these techniques to edit iPSCs proved much more difficult. Put simply, iPS cells don’t like to be genetically manipulated. Early iPS cell editing experiments were often inefficient or unsuccessful, not to mention extremely time-consuming.
One of the factors contributing to the resistance of iPSCs to genetic manipulation is that homology-directed repair (HDR) occurs even less frequently in iPS cells compared to immortal cell lines. Attempts to knock-in genes or correct mutations in iPS cells were largely unsuccessful because HDR was not occurring at high enough rates to repair the double-stranded breaks caused by CRISPR-Cas9. Performing multiple edits in iPSCs, which is often necessary to create models of multifactorial inheritance disorders, is even more challenging.
Solution: Since HDR only occurs at brief stages in the life cycle of cells, editing and reprogramming methods can be optimized by synchronizing the cell cycle in populations of iPSCs. This is usually achieved through methods like serum starvation when reprogramming iPSCs. However, a 2019 study screened small molecule drugs to identify a target that could synchronize iPSCs without affecting their unique characteristics.
Fortunately, it is much easier to edit iPSCs using CRISPR-Cas9 technology rather than earlier genome engineering technologies like TALENs and ZFNs. A key method to increase the likelihood of HDR for generating CRISPR iPS cells is by inhibiting the non-homologous end-joining (NHEJ) pathway, which is the more common DNA repair mechanism. A 2018 study found that by combining several different small molecule NHEJ inhibitors, they could achieve up to a 7.2 increase in CRISPR editing efficiencies in iPSCs.
Low editing efficienciesiPSC reprogramming is difficult to begin with, and achieving high efficiencies of gene editing in the cells is yet another obstacle. This is due to several reasons, including the PAM recognition of the Cas nuclease used for editing and guide RNAs, which require careful design.
Solutions: Improvements to the CRISPR system which have enabled the successful editing of iPSCs include the use of different Cas nucleases, such as Staphylococcus aureus Cas9 (SaCas9), and engineering the original Streptococcus pyogenes Cas9 (SpCas9) to recognize different protospacer adjacent motif (PAM) sequences.
Using high-quality, synthetic guide RNA and donor DNA templates also improves CRISPR editing efficiencies in iPS cells. Often, the addition of chemical modifications to guide RNAs is also necessary for iPSCs to be edited successfully. Chemical modifications, such as the addition of 2’-O-methyl and 3’ phosphorothioate on terminal nucleotides of synthetic guide RNAs, have been demonstrated to improve editing efficiencies significantly, helping the sgRNA to remain stable and protected from exonuclease damage.
iPSC reprogramming is difficult to begin with, and achieving high efficiencies of gene editing in the cells is yet another obstacle. This is due to several reasons, including the PAM recognition of the Cas nuclease used for editing and guide RNAs, which require careful design.
Solutions: Improvements to the CRISPR system which have enabled the successful editing of iPSCs include the use of different Cas nucleases, such as Staphylococcus aureus Cas9 (SaCas9), and engineering the original Streptococcus pyogenes Cas9 (SpCas9) to recognize different protospacer adjacent motif (PAM) sequences.
Using high-quality, synthetic guide RNA and donor DNA templates also improves CRISPR editing efficiencies in iPS cells. Often, the addition of chemical modifications to guide RNAs is also necessary for iPSCs to be edited successfully. Chemical modifications, such as the addition of 2’-O-methyl and 3’ phosphorothioate on terminal nucleotides of synthetic guide RNAs, have been demonstrated to improve editing efficiencies significantly, helping the sgRNA to remain stable and protected from exonuclease damage.
Time-consuming clonal expansion stepsAfter editing has been performed, the next critical stage of any CRISPR iPS cell experiment is to select individual clones and expand them before testing to confirm editing was successful. This process is laborious and time-consuming. For iPS cell editing, however, the process must be performed twice: once to reprogram the cells and select those that were successful, and again to edit the reprogrammed cells and select those with the desired edit.
Solutions: To avoid two stages of clonal expansion and the associated difficulties, many studies now combine the reprogramming step with the editing step. There are also several optimized protocols available for the expansion of CRISPR iPS cell clones, making the process easier and more efficient.
After editing has been performed, the next critical stage of any CRISPR iPS cell experiment is to select individual clones and expand them before testing to confirm editing was successful. This process is laborious and time-consuming. For iPS cell editing, however, the process must be performed twice: once to reprogram the cells and select those that were successful, and again to edit the reprogrammed cells and select those with the desired edit.
Solutions: To avoid two stages of clonal expansion and the associated difficulties, many studies now combine the reprogramming step with the editing step. There are also several optimized protocols available for the expansion of CRISPR iPS cell clones, making the process easier and more efficient.
Consistency and translational issues of CRISPR iPS cell therapyiPS cell lines from different origins have natural genetic and phenotypic variability, both genetically and in their ability to differentiate into other cell types. A study suggested that different iPS lines may differ by millions of single nucleotide polymorphisms (SNPs), and this variation leads to different experimental outcomes.
Since experiments must be highly replicated with consistent results to be considered for clinical trials in human patients, this variation has become a major stumbling block in many fields of research. Off-target editing is also a concern for the clinical use of CRISPR-edited iPS cells; any off-target cleavage of Cas9 could have potentially dangerous consequences for patients.
Solutions: One of the key improvements in the safety of CRISPR-Cas9 gene editing for use in clinical trials is the engineering of high-fidelity variants of Cas9. Cell and gene therapy studies often use these engineered variants because they have a much cleaner and safer profile, with very minimal off-target activity.
There are also improved methods for detecting off-target edits, including whole-genome sequencing, which can be analyzed in-depth by programs such as Synthego’s Inference of CRISPR Edits (ICE), CRISPResso, or OffScan. You can read more about the different computational tools for analyzing CRISPR experiments in this recent review.
iPS cell lines from different origins have natural genetic and phenotypic variability, both genetically and in their ability to differentiate into other cell types. A study suggested that different iPS lines may differ by millions of single nucleotide polymorphisms (SNPs), and this variation leads to different experimental outcomes.
Since experiments must be highly replicated with consistent results to be considered for clinical trials in human patients, this variation has become a major stumbling block in many fields of research. Off-target editing is also a concern for the clinical use of CRISPR-edited iPS cells; any off-target cleavage of Cas9 could have potentially dangerous consequences for patients.
Solutions: One of the key improvements in the safety of CRISPR-Cas9 gene editing for use in clinical trials is the engineering of high-fidelity variants of Cas9. Cell and gene therapy studies often use these engineered variants because they have a much cleaner and safer profile, with very minimal off-target activity.
There are also improved methods for detecting off-target edits, including whole-genome sequencing, which can be analyzed in-depth by programs such as Synthego’s Inference of CRISPR Edits (ICE), CRISPResso, or OffScan. You can read more about the different computational tools for analyzing CRISPR experiments in this recent review.
Synthego Solution: CRISPR-Cas9 iPSCs
It’s possible that you don’t have the time, equipment, or lab capacity to create your own CRISPR iPS cells. Fortunately, Synthego can help! Leveraging our automation capabilities, our workflow for generating CRISPR iPS cells includes several steps that ensure high editing efficiencies while maintaining a high standard of quality.
As seen in the illustration below, Synthego employs a streamlined workflow that can produce pools and clones of edited iPS cells. Edited pools are cell populations that contain both edited and wild-type cells. Clones are populations of genetically identical cells that contain the desired edit.
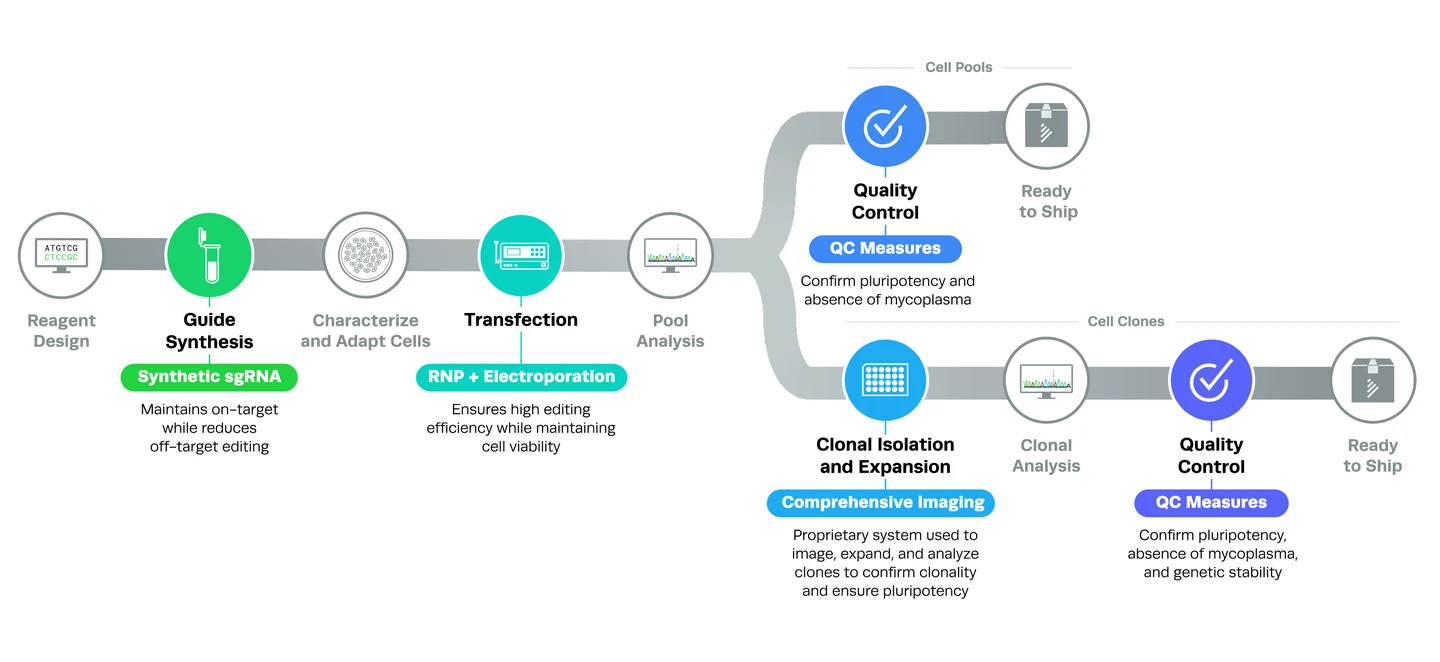
Several steps in the editing process enable Synthego to produce CRISPR iPS cells of the highest quality. Let’s take a closer look at how it works in this section.
High-quality synthetic guide RNAs and chemical modificationsAll guide RNAs are produced synthetically and contain chemical modifications that increase stability and lower immunogenicity. For more information, you can read our complete guide to understanding CRISPR sgRNA or check out our synthetic sgRNA kits.
All guide RNAs are produced synthetically and contain chemical modifications that increase stability and lower immunogenicity. For more information, you can read our complete guide to understanding CRISPR sgRNA or check out our synthetic sgRNA kits.
Optimized transfection protocolsTo create CRISPR iPS cells, the guide RNA and Cas9 nuclease are introduced to cells as ribonucleoproteins (RNPs) using electroporation. Because RNPs cut the DNA quickly but for a limited period of time, the risk of off-target editing is decreased. Electroporation utilizes electrical impulses to introduce RNPs into cells and enables high editing efficiencies in a variety of cell types.
To create CRISPR iPS cells, the guide RNA and Cas9 nuclease are introduced to cells as ribonucleoproteins (RNPs) using electroporation. Because RNPs cut the DNA quickly but for a limited period of time, the risk of off-target editing is decreased. Electroporation utilizes electrical impulses to introduce RNPs into cells and enables high editing efficiencies in a variety of cell types.
Quality control checks for CRISPR iPS cellsStringent quality control checks are integral parts of the workflow for both pools and clones of CRISPR iPS cells. Edited pools are checked for purity and pluripotency, and clones are checked for purity, pluripotency, and genomic stability (karyotyping).
Stringent quality control checks are integral parts of the workflow for both pools and clones of CRISPR iPS cells. Edited pools are checked for purity and pluripotency, and clones are checked for purity, pluripotency, and genomic stability (karyotyping).
Clonal isolation and expansion methodsSynthego uses proprietary imaging technology to ensure that each CRISPR iPSC clone originates from a single cell and that selected clones are pluripotent. For more information, you can download our limiting dilution and clonal expansion protocol.
We hope you enjoyed this updated iPS cell blog! Although CRISPR iPS cells offer enormous potential for advancement in the biomedical field, the difficulties associated with their handling can serve as a deterrent to their use. On top of that, iPSC gene editing while keeping the cells happy is an even greater hurdle. Let Synthego handle the tedious work - we deliver CRISPR iPS cells with your desired edit, guaranteed!
Check out our product page to learn more about our CRISPR iPS cell workflow and offerings!
Synthego uses proprietary imaging technology to ensure that each CRISPR iPSC clone originates from a single cell and that selected clones are pluripotent. For more information, you can download our limiting dilution and clonal expansion protocol.
We hope you enjoyed this updated iPS cell blog! Although CRISPR iPS cells offer enormous potential for advancement in the biomedical field, the difficulties associated with their handling can serve as a deterrent to their use. On top of that, iPSC gene editing while keeping the cells happy is an even greater hurdle. Let Synthego handle the tedious work - we deliver CRISPR iPS cells with your desired edit, guaranteed!
Check out our product page to learn more about our CRISPR iPS cell workflow and offerings!