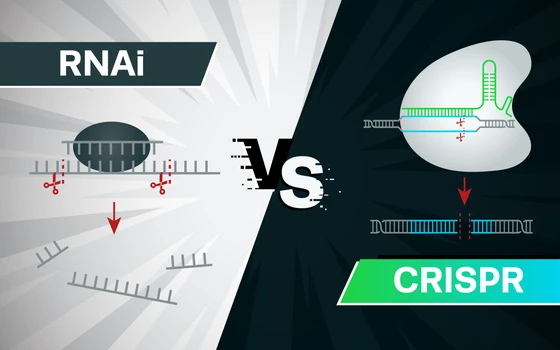
The process for drug discovery and therapeutic development heavily relies on correlating genotype to phenotype. The best way to achieve this is to disrupt gene function and analyze its phenotypic. Researchers can experimentally regulate gene expression and interrogate gene function either at the translational level or at the genetic level using two biological tools, RNAi and CRISPR, respectively.
How do these methods differ from one another? Is one more suitable than the other for certain experiments? In this article, we will answer these questions by comparing the mechanisms of action, advantages, and limitations of RNAi and CRISPR to guide you to an informed decision regarding the best technique to use in your experiments.
RNAi: The Knockdown Pioneer
In this section, we will focus on the history, mechanism, and experimental workflow of RNAi.
History of RNAiIn 1990, researchers noticed for the first time that RNA could potentially suppress gene expression in plants. The underlying mechanism of this “erratic and reversible” gene suppression was not clear. It wasn’t until almost a decade later when Andrew Fire and Craig Mello showed in the worm Caenorhabditis elegans that double-stranded RNA (dsRNA)—but not single-stranded RNA (ssRNA)—was involved in gene silencing. Moreover, their report established that RNA interference (RNAi) occurs in a sequence-specific manner. This finding triggered a series of studies unraveling the detailed mechanism of RNAi over the years. In 2006, Fire and Mello were awarded the Nobel Prize for Physiology or Medicine for their seminal work.
In 1990, researchers noticed for the first time that RNA could potentially suppress gene expression in plants. The underlying mechanism of this “erratic and reversible” gene suppression was not clear. It wasn’t until almost a decade later when Andrew Fire and Craig Mello showed in the worm Caenorhabditis elegans that double-stranded RNA (dsRNA)—but not single-stranded RNA (ssRNA)—was involved in gene silencing. Moreover, their report established that RNA interference (RNAi) occurs in a sequence-specific manner. This finding triggered a series of studies unraveling the detailed mechanism of RNAi over the years. In 2006, Fire and Mello were awarded the Nobel Prize for Physiology or Medicine for their seminal work.
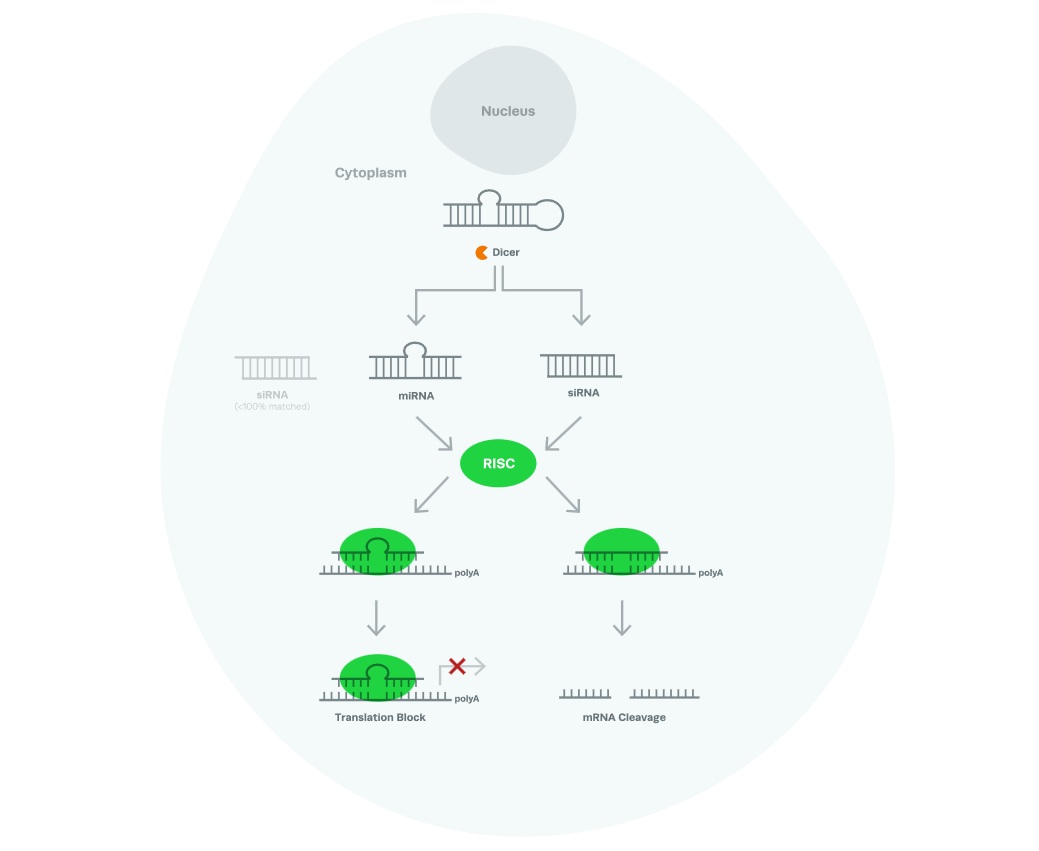
RNAi MechanismAlthough researchers demonstrated RNAi functionality using exogenous dsRNAs, RNA interference is also triggered by the endogenously present single-stranded hairpin microRNAs (miRNA) in cells, small interfering RNAs (siRNA), or short hairpin RNAs (shRNA).
The primary function of natural RNA interference is to regulate gene expression. In some cases, RNAi can also confer resistance to virus or other pathogen infection.
The dsRNA that enters the cell (or the existing pre-miRNA in cells) is cleaved into smaller RNA fragments of around 21 nucleotides in length by an endonuclease, Dicer. These RNAs associate with the RNA-induced silencing complex (RISC), the antisense strand is separated from the sense strand, and are targeted to their complementary mRNA.
Following association of the siRNA or miRNA with their target, argonaute, a protein from the RISC complex, cleaves the mRNA and inhibits expression of the protein that it encodes. If the sequences of siRNA or miRNA do not perfectly match the sequence of the mRNA, then the mRNA is not cleaved, but translation is still stalled, as the RISC complex physically blocks the mRNA.
Although researchers demonstrated RNAi functionality using exogenous dsRNAs, RNA interference is also triggered by the endogenously present single-stranded hairpin microRNAs (miRNA) in cells, small interfering RNAs (siRNA), or short hairpin RNAs (shRNA).
The primary function of natural RNA interference is to regulate gene expression. In some cases, RNAi can also confer resistance to virus or other pathogen infection.
The dsRNA that enters the cell (or the existing pre-miRNA in cells) is cleaved into smaller RNA fragments of around 21 nucleotides in length by an endonuclease, Dicer. These RNAs associate with the RNA-induced silencing complex (RISC), the antisense strand is separated from the sense strand, and are targeted to their complementary mRNA.
Following association of the siRNA or miRNA with their target, argonaute, a protein from the RISC complex, cleaves the mRNA and inhibits expression of the protein that it encodes. If the sequences of siRNA or miRNA do not perfectly match the sequence of the mRNA, then the mRNA is not cleaved, but translation is still stalled, as the RISC complex physically blocks the mRNA.
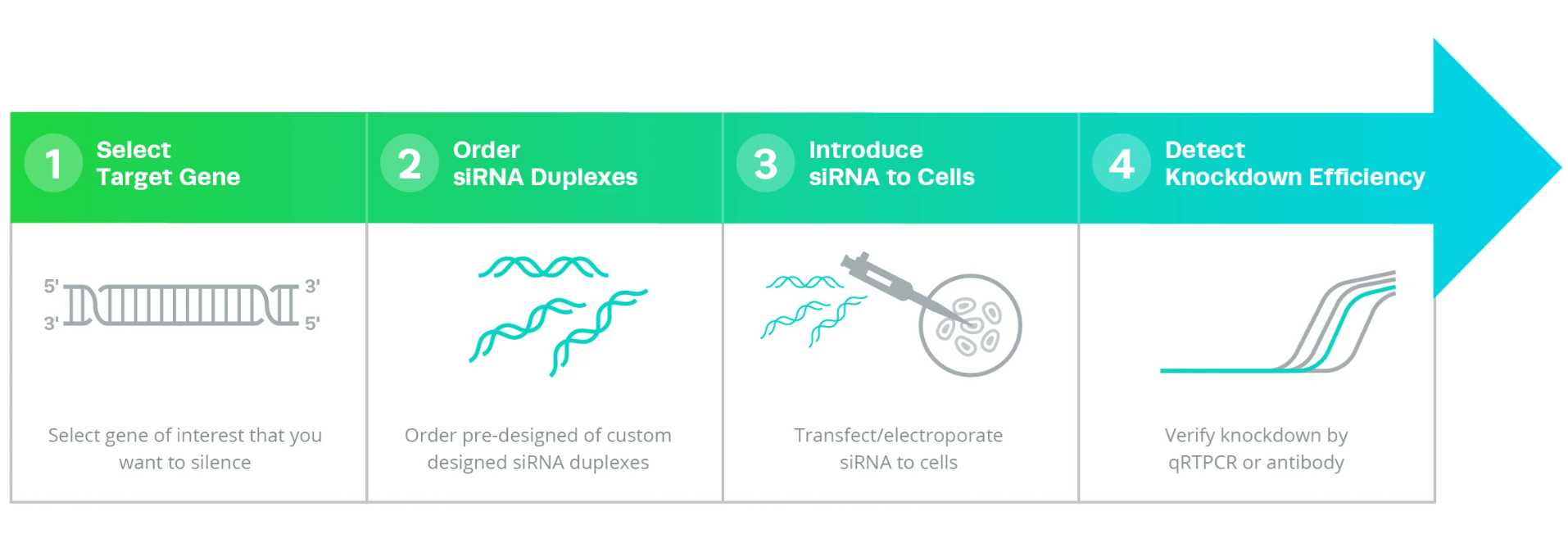
RNAi Experimental WorkflowThe first step in RNAi experiments is designing the siRNAs or miRNAs. Ideally, highly specific siRNAs that target only the intended genes are desired.
Once the specific siRNAs or miRNAs are designed, they can be introduced into cells using plasmid vectors, synthetic siRNA, PCR products, or in vitro transcribed siRNAs. One advantage of RNAi is that animal cells naturally possess the endogenous machinery (Dicer and RISC) essential for the process. Therefore, few components need to be delivered inside the cells, making the experimental process relatively easy to execute.
In the last step, the efficiency of gene silencing is generally determined by measuring the mRNA transcript levels using methods such as quantitative RT-PCR, measuring protein levels using immunoblotting or immunofluorescence experiments, or by monitoring obvious phenotypic changes.
The first step in RNAi experiments is designing the siRNAs or miRNAs. Ideally, highly specific siRNAs that target only the intended genes are desired.
Once the specific siRNAs or miRNAs are designed, they can be introduced into cells using plasmid vectors, synthetic siRNA, PCR products, or in vitro transcribed siRNAs. One advantage of RNAi is that animal cells naturally possess the endogenous machinery (Dicer and RISC) essential for the process. Therefore, few components need to be delivered inside the cells, making the experimental process relatively easy to execute.
In the last step, the efficiency of gene silencing is generally determined by measuring the mRNA transcript levels using methods such as quantitative RT-PCR, measuring protein levels using immunoblotting or immunofluorescence experiments, or by monitoring obvious phenotypic changes.
CRISPR: Knockouts For Loss-of-Function ExperimentsLearn about the discovery of CRISPR, understand its mechanism, and explore the experimental workflow in this section.
Learn about the discovery of CRISPR, understand its mechanism, and explore the experimental workflow in this section.
History of CRISPRThe foundational discoveries leading to the development of CRISPR-Cas9 technology date back to 1987, when researchers first identified palindromic segments of DNA in bacteria. At the time, the significance of these Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), was not known.
In fact, it wasn't until 2007 that researchers showed that CRISPR plays an important role in microbial innate immunity. Microbes, upon viral infection, deploy a special nuclease, directed by a guide RNA, to cleave specific sequences of the viral DNA. The excised DNA fragment may be stored to retain a genetic memory and disable future infections, similar to the array of antigens stored in our immune system for cellular defense against pathogens. In 2012, research groups of Doudna and Charpentier collaboratively deduced the cleavage mechanism of RNA-guided Cas9 and predicted its potential use in programmed genome editing.
In 2013, Feng Zhang’s group used the CRISPR system for genome editing in eukaryotic cells (human and mouse) for the first time. Thereafter, CRISPR has been harnessed as a common tool for editing genomes of various organisms in research projects across the globe.
The foundational discoveries leading to the development of CRISPR-Cas9 technology date back to 1987, when researchers first identified palindromic segments of DNA in bacteria. At the time, the significance of these Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), was not known.
In fact, it wasn't until 2007 that researchers showed that CRISPR plays an important role in microbial innate immunity. Microbes, upon viral infection, deploy a special nuclease, directed by a guide RNA, to cleave specific sequences of the viral DNA. The excised DNA fragment may be stored to retain a genetic memory and disable future infections, similar to the array of antigens stored in our immune system for cellular defense against pathogens. In 2012, research groups of Doudna and Charpentier collaboratively deduced the cleavage mechanism of RNA-guided Cas9 and predicted its potential use in programmed genome editing.
In 2013, Feng Zhang’s group used the CRISPR system for genome editing in eukaryotic cells (human and mouse) for the first time. Thereafter, CRISPR has been harnessed as a common tool for editing genomes of various organisms in research projects across the globe.
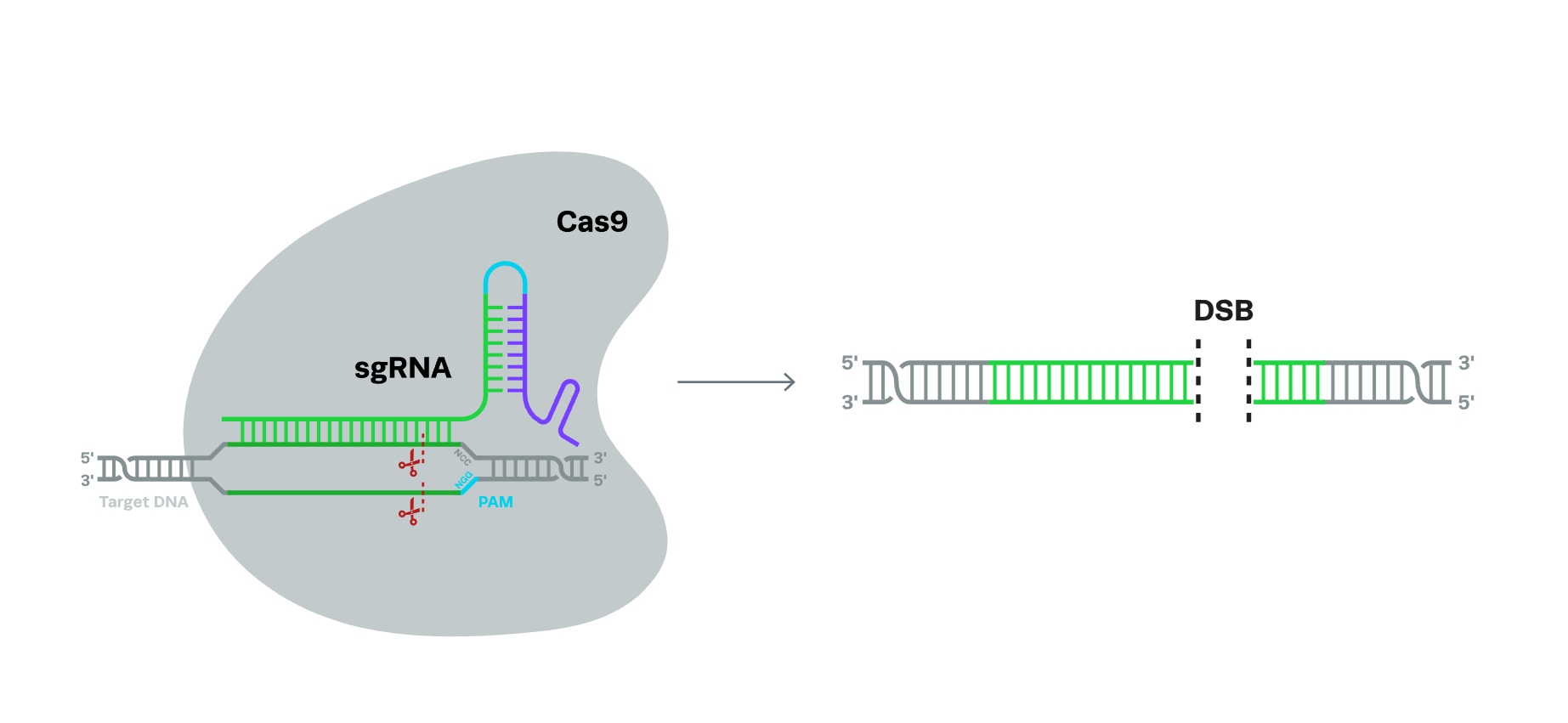
CRISPR MechanismCRISPR-based genome editing requires two components: a guide RNA and a CRISPR-associated endonuclease protein (Cas). The guide RNA, analogous to a GPS system, directs the Cas nuclease to the specific target DNA sequence, which then cuts the DNA at that site. The most commonly used nuclease, SpCas9, is the one isolated from the bacterium Streptococcus pyogenes.
The SpCas9 nuclease contains two protein lobes: a recognition lobe and a nuclease lobe. Upon binding the target DNA sequence with the help of the guide RNA, the recognition lobe interacts with the DNA strand to double-check for complementarity. The nuclease lobe, similar to a pair of molecular scissors, then creates a double-strand break (DSB) in the target DNA.
Once a DSB is created in the DNA, the cell tries to repair it via non-homologous end to end joining (NHEJ), which is a quick-fix repair mechanism for ligation of the blunt ends of DNA and is prone to errors. The process often results in insertions or deletion of bases (indels), which can lead to protein disruption, and is the preferred pathway for knocking out a particular gene.
Note that while NHEJ yields knockouts, CRISPR can also be used to knock-ins desired sequences at specific loci in the cellular genome via the homology-directed pathway, HDR.
CRISPR-based genome editing requires two components: a guide RNA and a CRISPR-associated endonuclease protein (Cas). The guide RNA, analogous to a GPS system, directs the Cas nuclease to the specific target DNA sequence, which then cuts the DNA at that site. The most commonly used nuclease, SpCas9, is the one isolated from the bacterium Streptococcus pyogenes.
The SpCas9 nuclease contains two protein lobes: a recognition lobe and a nuclease lobe. Upon binding the target DNA sequence with the help of the guide RNA, the recognition lobe interacts with the DNA strand to double-check for complementarity. The nuclease lobe, similar to a pair of molecular scissors, then creates a double-strand break (DSB) in the target DNA.
Once a DSB is created in the DNA, the cell tries to repair it via non-homologous end to end joining (NHEJ), which is a quick-fix repair mechanism for ligation of the blunt ends of DNA and is prone to errors. The process often results in insertions or deletion of bases (indels), which can lead to protein disruption, and is the preferred pathway for knocking out a particular gene.
Note that while NHEJ yields knockouts, CRISPR can also be used to knock-ins desired sequences at specific loci in the cellular genome via the homology-directed pathway, HDR.
CRISPR Experimental workflowOne of the most critical steps of a CRISPR experiment is designing efficient and specific guide RNAs, which is facilitated by the availability of state-of-the-art design tools.
Choosing the delivery format of the CRISPR components is the next step in the CRISPR workflow. Transfecting plasmids bearing the guide RNA sequence and nuclease encoding the Cas nuclease sequence has been conventionally used in genome engineering. In vitro transcribed RNAs (IVT) have also been used widely in CRISPR experiments.
However, both these delivery systems suffer from low efficiency and labor-intensive methods. The availability of economical, high-quality synthetic guide RNA allows the delivery of CRISPR components complexed in a ribonucleoprotein (RNP) format, which enables the highest editing efficiencies and the most reproducible CRISPR results. The RNP format is now the preferred choice of many researchers for genome engineering experiments.
Once the guide RNA is transfected into cells, the editing efficiency is analyzed using tools such as ICE.
One of the most critical steps of a CRISPR experiment is designing efficient and specific guide RNAs, which is facilitated by the availability of state-of-the-art design tools.
Choosing the delivery format of the CRISPR components is the next step in the CRISPR workflow. Transfecting plasmids bearing the guide RNA sequence and nuclease encoding the Cas nuclease sequence has been conventionally used in genome engineering. In vitro transcribed RNAs (IVT) have also been used widely in CRISPR experiments.
However, both these delivery systems suffer from low efficiency and labor-intensive methods. The availability of economical, high-quality synthetic guide RNA allows the delivery of CRISPR components complexed in a ribonucleoprotein (RNP) format, which enables the highest editing efficiencies and the most reproducible CRISPR results. The RNP format is now the preferred choice of many researchers for genome engineering experiments.
Once the guide RNA is transfected into cells, the editing efficiency is analyzed using tools such as ICE.

Comparison Between CRISPR and RNAi
Choosing the Right Method for Gene SilencingThe primary difference between RNAi and CRISPR is that RNAi reduces gene expression at the mRNA level (knockdown), while CRISPR completely and permanently silences the gene at the DNA level (knockout).
As gene silencing methods, both knockouts and knockdowns have their own pros and cons. Knockouts of essential genes are lethal, providing only partial information regarding gene function in studies where the gene of interest plays a crucial role in the survival of the organism. In such cases, incomplete gene knockdown can provide a better understanding of the gene effect on phenotype because the effects of reducing protein levels to different extents can be studied.
Moreover, the reversible nature of knockdowns makes it possible to verify the phenotypic effect by restoring protein expression to normal in the same cells. Importantly, since a knockdown is transient, it can be a safer option than permanent genome editing. These features made RNAi an instant hit with researchers for transiently blocking gene expression.
On the other hand, knockouts are effective in completely blocking protein expression, eliminating any confounding effects from remnant low levels of protein expression post knockdown. As CRISPR became popular for its ease of genetic editing, variations in the method and new versions of CRISPR-associated nucleases enabled researchers to use CRISPR for applications beyond gene knockouts.
For instance, CRISPRi allows the silencing of genes without permanently knocking out the gene. This is achieved using a dead Cas9 nuclease that physically blocks RNA polymerase and inhibits gene transcription or by editing gene regulators to modulate gene expression. Although the mechanism is different from knocking out genes, the inhibition still occurs at the DNA level.
Recently, researchers have developed nuclease variants that can target RNA instead of DNA, yielding an outcome similar to RNAi.
The primary difference between RNAi and CRISPR is that RNAi reduces gene expression at the mRNA level (knockdown), while CRISPR completely and permanently silences the gene at the DNA level (knockout).
As gene silencing methods, both knockouts and knockdowns have their own pros and cons. Knockouts of essential genes are lethal, providing only partial information regarding gene function in studies where the gene of interest plays a crucial role in the survival of the organism. In such cases, incomplete gene knockdown can provide a better understanding of the gene effect on phenotype because the effects of reducing protein levels to different extents can be studied.
Moreover, the reversible nature of knockdowns makes it possible to verify the phenotypic effect by restoring protein expression to normal in the same cells. Importantly, since a knockdown is transient, it can be a safer option than permanent genome editing. These features made RNAi an instant hit with researchers for transiently blocking gene expression.
On the other hand, knockouts are effective in completely blocking protein expression, eliminating any confounding effects from remnant low levels of protein expression post knockdown. As CRISPR became popular for its ease of genetic editing, variations in the method and new versions of CRISPR-associated nucleases enabled researchers to use CRISPR for applications beyond gene knockouts.
For instance, CRISPRi allows the silencing of genes without permanently knocking out the gene. This is achieved using a dead Cas9 nuclease that physically blocks RNA polymerase and inhibits gene transcription or by editing gene regulators to modulate gene expression. Although the mechanism is different from knocking out genes, the inhibition still occurs at the DNA level.
Recently, researchers have developed nuclease variants that can target RNA instead of DNA, yielding an outcome similar to RNAi.
Comparing Specificity of CRISPR and RNAiOne of the biggest limitations of the RNAi silencing method is that it suffers from high off-target effects. Silencing unintended RNA targets results in modified phenotypes and is therefore detrimental for gene function screening experiments.
The off-target effects in RNAi may be of two types: sequence-independent and sequence-dependent. For instance, multiple studies have shown that siRNAs trigger an interferon activated pathway in certain cell types in a sequence-independent manner, resulting in increased expression of interferon-regulated genes. In 2003, research showed for the first time that siRNA also targeted sequences with limited complementarity. Even today, sequence-based off-target effects remain the most challenging issue in RNAi experiments.
The CRISPR system initially also had some sequence-specific off-target effects. However, in a short time timespan, the technology has advanced rapidly in multiple areas. Efficient design tools enabled finding guide RNAs that exhibit minimal off-target effects. The introduction of sgRNA and further chemically modified sgRNAs have also greatly contributed to reducing the off-target effects relative to plasmid and IVT-derived guide RNAs.
Although optimizing siRNA design, concentrations, and chemical modifications have decreased some of the off-target effects of RNAi, a recent comparative study showed that CRISPR has far fewer off-target effects than RNAi.
Thus, CRISPR is likely to continue its rapid growth and replace RNAi for most research applications, including clinical trials.
One of the biggest limitations of the RNAi silencing method is that it suffers from high off-target effects. Silencing unintended RNA targets results in modified phenotypes and is therefore detrimental for gene function screening experiments.
The off-target effects in RNAi may be of two types: sequence-independent and sequence-dependent. For instance, multiple studies have shown that siRNAs trigger an interferon activated pathway in certain cell types in a sequence-independent manner, resulting in increased expression of interferon-regulated genes. In 2003, research showed for the first time that siRNA also targeted sequences with limited complementarity. Even today, sequence-based off-target effects remain the most challenging issue in RNAi experiments.
The CRISPR system initially also had some sequence-specific off-target effects. However, in a short time timespan, the technology has advanced rapidly in multiple areas. Efficient design tools enabled finding guide RNAs that exhibit minimal off-target effects. The introduction of sgRNA and further chemically modified sgRNAs have also greatly contributed to reducing the off-target effects relative to plasmid and IVT-derived guide RNAs.
Although optimizing siRNA design, concentrations, and chemical modifications have decreased some of the off-target effects of RNAi, a recent comparative study showed that CRISPR has far fewer off-target effects than RNAi.
Thus, CRISPR is likely to continue its rapid growth and replace RNAi for most research applications, including clinical trials.
High-Throughput Genetic Screening: CRISPR vs. RNAiAlthough CRISPR and RNAi differ in their mechanisms of action, both methods can independently (or in combination) be used to accomplish certain goals. A perfect example is their application in high-throughput genetic screening. The approach involves disrupting multiple genes in cells and analyzing the resulting phenotypic effects. Results are analyzed by often investigating detectable phenotypic markers such as resistance to antibiotics, activation of a fluorescent marker, or faster cell growth allowed correlating gene and function.
Before the discovery of CRISPR, RNAi libraries were commonly used for screening gene function. But now CRISPR has emerged as an important tool in target identification and validation studies, particularly with the availability of arrayed synthetic sgRNA libraries. The arrayed format enables easy data deconvolution as compared to pool format and synthetic sgRNA achieves consistently high editing efficiencies.
Synthego’s Arrayed CRISPR Libraries enable confident screening with minimum off-targets and false negatives. Read how these libraries benefitted the lab Kaylene Simpson, Head of VCFG, Peter MacCallum Cancer Center, in this case study.
Although CRISPR and RNAi differ in their mechanisms of action, both methods can independently (or in combination) be used to accomplish certain goals. A perfect example is their application in high-throughput genetic screening. The approach involves disrupting multiple genes in cells and analyzing the resulting phenotypic effects. Results are analyzed by often investigating detectable phenotypic markers such as resistance to antibiotics, activation of a fluorescent marker, or faster cell growth allowed correlating gene and function.
Before the discovery of CRISPR, RNAi libraries were commonly used for screening gene function. But now CRISPR has emerged as an important tool in target identification and validation studies, particularly with the availability of arrayed synthetic sgRNA libraries. The arrayed format enables easy data deconvolution as compared to pool format and synthetic sgRNA achieves consistently high editing efficiencies.
Synthego’s Arrayed CRISPR Libraries enable confident screening with minimum off-targets and false negatives. Read how these libraries benefitted the lab Kaylene Simpson, Head of VCFG, Peter MacCallum Cancer Center, in this case study.
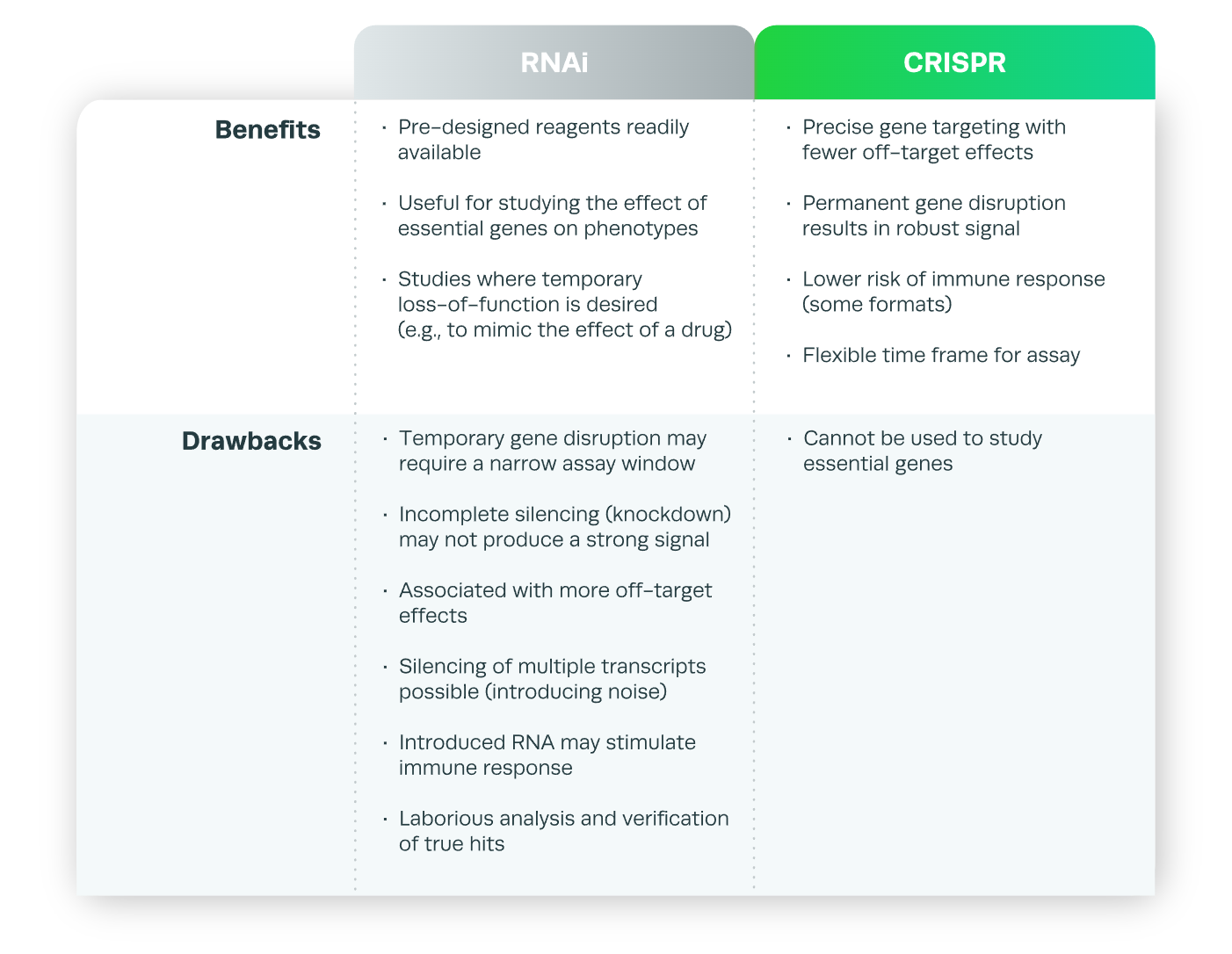
In summary, although RNAi was adopted as a gene-silencing technique first, CRISPR has several advantages over RNAi, as listed in Table.1. If you are looking for further resources to better understand libraries, check out our libraries resources section.
Arrayed CRISPR Screening Libraries
Synthego’s multi-guide arrayed CRISPR library design ensures incredibly high knockout efficiency so you can be confident in your hits.