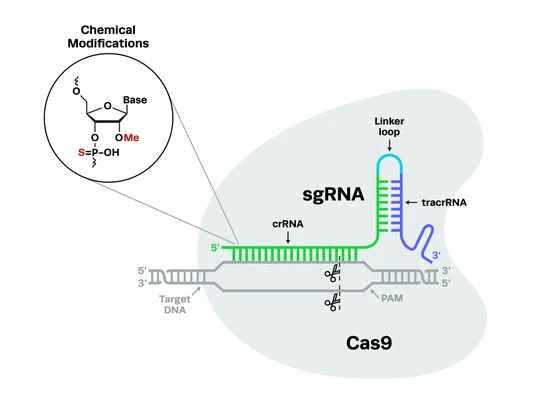
Since the advent of CRISPR-Cas9 genome engineering technology, one of the key limiting factors has been the delivery of CRISPR components into cells. With so many different targets and applications for CRISPR, from studies of cell lines grown in vitro to clinical trials, it is only fitting that there is a wide variety of CRISPR delivery systems available. This includes CRISPR viral delivery, non-viral methods, and physical cell manipulation techniques. This article is a deep dive into the world of CRISPR delivery and CRISPR cargo, including the pros and cons associated with each delivery method, CRISPR delivery challenges, and a discussion of nascent CRISPR delivery technologies.
How Does CRISPR Delivery Work?
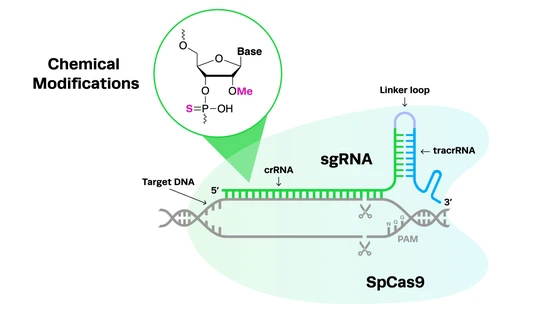
CRISPR delivery methods involve both the vehicle (the method of delivery into cells) and cargo (Cas nuclease, guide RNA, and potentially a DNA donor template for homology-directed repair). CRISPR delivery vehicles fall into three categories: viral, non-viral, and physical. The delivery vehicle will determine whether the Cas nuclease can be delivered as DNA, mRNA, or protein.
Each CRISPR delivery vehicle, and the combination of vehicle with cargo, has its own advantages and disadvantages, and will have an effect on the safety and efficacy of the CRISPR experiment or treatment.
The in vivo, ex vivo, or in vitro status of the experiment will also determine the type of cargo and delivery system that can be used, as will the cell type that is being targeted. Delivery of CRISPR-Cas9 for therapeutic genome editing presents an enormous challenge, as it must be as risk-averse and efficient as possible, with high specificity.
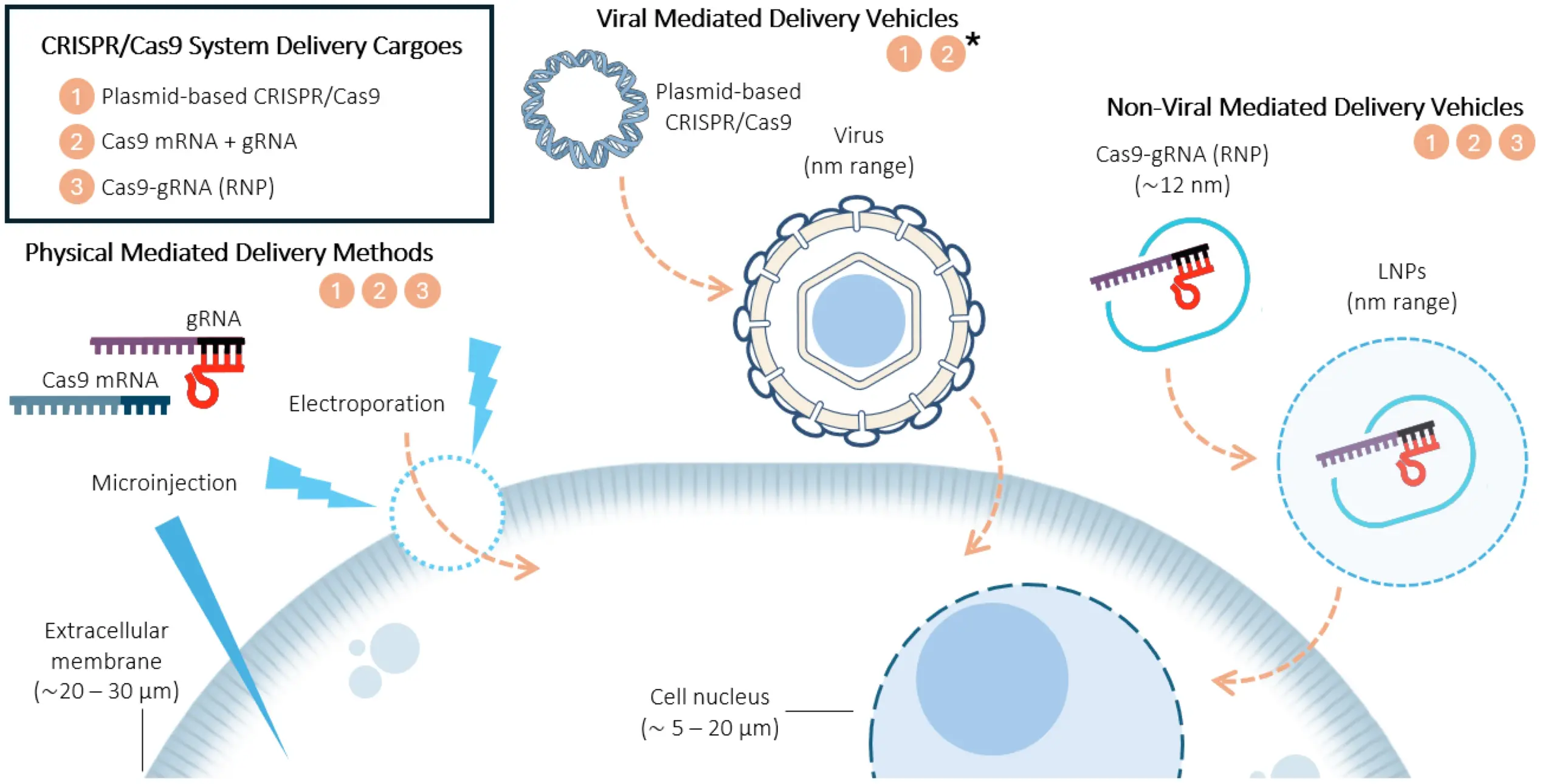
Types of CRISPR cargo
Types of cargo for CRISPR delivery can take three possible forms:
- DNA: a DNA plasmid containing the sequences for both Cas nuclease and guide RNA.
- RNA: mRNA for translation of the Cas protein, with a separate guide RNA.
- Protein: a ribonucleoprotein (RNP) complex, made up of the Cas protein and a guide RNA.
CRISPR cargo can be tricky to get into cells, particularly when working with cells that are not amenable to transfection. DNA plasmids containing the sequences for the Cas and guide RNA were the most widely used cargo in the early years of CRISPR delivery systems. However, there are several problems associated with plasmid cargos, including cytotoxicity, variable editing efficiency, prolonged activity, and subsequent off-target effects, and timing issues.
CRISPR RNP delivery has been widely adopted, as RNP complexes are immediately active, can be delivered via several different vehicles, and have increased precision and reduced off-target effects.
CRISPR delivery methods for cell and gene therapy
Each of the three categories of CRISPR delivery methods is further divided into several specific vehicles. Some of these were adopted early in the history of CRISPR, while others are more recent discoveries, based on a need to develop safer, more efficient, or more targeted delivery methods, or for use with specific types of cells.
In this section, we will learn about the specific CRISPR delivery vehicles for viral, non-viral, and physical methods as they apply to cell and gene therapy applications, and discuss the advantages and disadvantages of each.
For more information, including off-target effects and delivery to specific types of cells and cellular compartments, you can watch this video of the CRISPR in Genome Biology session from World CRISPR Day below.
CRISPR viral deliveryCRISPR viral delivery can be achieved through the use of adeno-associated viral vectors (AAVVs), full-size adenoviral vectors (AdVs), and lentiviral vectors (LVs). Viral vectors are particularly popular for in vivo CRISPR experiments and clinical trials, however, they also lend themselves to ex vivo and in vitro studies, and are useful in studies requiring long-term expression of CRISPR components.
Researchers have also more recently developed virus-like particles (VLPs), which have some of the advantages of viral delivery vehicles without some of their disadvantages. HEK293T cells are typically used to create viral particles containing the CRISPR cargo for all three of the CRISPR viral delivery methods described here. The viral particles are then used to infect the target cells, resulting in the continued production of CRISPR cargo within the host.
CRISPR viral delivery can be achieved through the use of adeno-associated viral vectors (AAVVs), full-size adenoviral vectors (AdVs), and lentiviral vectors (LVs). Viral vectors are particularly popular for in vivo CRISPR experiments and clinical trials, however, they also lend themselves to ex vivo and in vitro studies, and are useful in studies requiring long-term expression of CRISPR components.
Researchers have also more recently developed virus-like particles (VLPs), which have some of the advantages of viral delivery vehicles without some of their disadvantages. HEK293T cells are typically used to create viral particles containing the CRISPR cargo for all three of the CRISPR viral delivery methods described here. The viral particles are then used to infect the target cells, resulting in the continued production of CRISPR cargo within the host.
Adeno-associated viral vectors (AAVVs or AAVs)AAVs are small (~20nm), non-pathogenic viruses. Because they are not known to cause disease and have such mild immune responses in humans, they quickly became one of the most popular CRISPR delivery methods, specifically in the development of cell and gene therapies.
- Advantages: AAV CRISPR delivery has two key advantages: the adeno-associated virus is not inserted into the host cell genome, and host immune responses are typically milder than when other viral delivery methods are used. For these reasons, AAVs have received FDA approval for the treatment of certain diseases, making them the most common method used for preclinical models of disease as well as for in vivo CRISPR delivery.
- Disadvantages: The use of AAVs is often limited by the size of cargo that can be packaged within them; their 4.7kb payload capacity makes them too small to carry the Cas nuclease, sgRNA, and in the case of gene correction or insertion, a donor template for repair. Several strategies have been developed to overcome this hurdle. The first is to use AAVs to deliver only the sgRNA into cells that have been previously edited to express the Cas nuclease. The second is to package the sgRNA and Cas nuclease into separate AAVs with unique tags, co-transfect them into cells, then screen cells for successful co-transfection.
Another strategy to circumvent the payload restriction of AAVs is to use different CRISPR systems for editing. Several smaller, naturally occurring Cas variants have been discovered, while some nucleases have been engineered to minimize their size and achieve higher editing efficiencies. For example, the traditionally-used SpCas9 is 1368 amino acids in length, while Synthego’s high-fidelity hfCas12Max nuclease is only 1080aa.
AAVs are small (~20nm), non-pathogenic viruses. Because they are not known to cause disease and have such mild immune responses in humans, they quickly became one of the most popular CRISPR delivery methods, specifically in the development of cell and gene therapies.
- Advantages: AAV CRISPR delivery has two key advantages: the adeno-associated virus is not inserted into the host cell genome, and host immune responses are typically milder than when other viral delivery methods are used. For these reasons, AAVs have received FDA approval for the treatment of certain diseases, making them the most common method used for preclinical models of disease as well as for in vivo CRISPR delivery.
- Disadvantages: The use of AAVs is often limited by the size of cargo that can be packaged within them; their 4.7kb payload capacity makes them too small to carry the Cas nuclease, sgRNA, and in the case of gene correction or insertion, a donor template for repair. Several strategies have been developed to overcome this hurdle. The first is to use AAVs to deliver only the sgRNA into cells that have been previously edited to express the Cas nuclease. The second is to package the sgRNA and Cas nuclease into separate AAVs with unique tags, co-transfect them into cells, then screen cells for successful co-transfection.
Another strategy to circumvent the payload restriction of AAVs is to use different CRISPR systems for editing. Several smaller, naturally occurring Cas variants have been discovered, while some nucleases have been engineered to minimize their size and achieve higher editing efficiencies. For example, the traditionally-used SpCas9 is 1368 amino acids in length, while Synthego’s high-fidelity hfCas12Max nuclease is only 1080aa.
Full-size adenoviral vectors (AdVs)AdVs are ubiquitous, with many serotypes present in the human population, causing diseases with varying degrees of severity. They can be used for CRISPR delivery, however, they are less suited to clinical applications than AAVs.
- Advantages: Similar to AAVVs, AdVs are not integrated into the host genome. Unlike AAVVs, however, they are not limited by size and can therefore carry much larger quantities of CRISPR cargo of up to 36kb. They can infect both dividing and non-dividing cells, can be grown in large quantities, and can be either replication-defective (RD) or replication-competent (RC). They can be modified to target specific cell types and to avoid immune responses.
- Disadvantages: AdVs can potentially cause undesirable immune responses and off-target effects. They can also result in damage to the host if they are inserted near key cellular proteins.
AdVs are ubiquitous, with many serotypes present in the human population, causing diseases with varying degrees of severity. They can be used for CRISPR delivery, however, they are less suited to clinical applications than AAVs.
- Advantages: Similar to AAVVs, AdVs are not integrated into the host genome. Unlike AAVVs, however, they are not limited by size and can therefore carry much larger quantities of CRISPR cargo of up to 36kb. They can infect both dividing and non-dividing cells, can be grown in large quantities, and can be either replication-defective (RD) or replication-competent (RC). They can be modified to target specific cell types and to avoid immune responses.
- Disadvantages: AdVs can potentially cause undesirable immune responses and off-target effects. They can also result in damage to the host if they are inserted near key cellular proteins.
Lentiviral vectors (LVs)Lentiviruses are retroviruses causing many serious diseases in animals and humans. LVs have a backbone which is a provirus of HIV, and they are commonly used for CRISPR delivery in in vitro studies and in animal models.
- Advantages: LVs can be pseudotyped with envelopes from naturally occurring or engineered viruses, meaning that their infectivity of specific cell lines can be altered to suit the target. Like AdVs, they are able to deliver any size of CRISPR cargo. They can also infect both dividing and non-dividing cells.
- Disadvantages: Unlike AAVVs and AdVs, LVs are inserted into the host genome. The presence of an HIV backbone means the chance of integration into the host genome cannot be completely avoided and this has associated safety implications.
Lentiviruses are retroviruses causing many serious diseases in animals and humans. LVs have a backbone which is a provirus of HIV, and they are commonly used for CRISPR delivery in in vitro studies and in animal models.
- Advantages: LVs can be pseudotyped with envelopes from naturally occurring or engineered viruses, meaning that their infectivity of specific cell lines can be altered to suit the target. Like AdVs, they are able to deliver any size of CRISPR cargo. They can also infect both dividing and non-dividing cells.
- Disadvantages: Unlike AAVVs and AdVs, LVs are inserted into the host genome. The presence of an HIV backbone means the chance of integration into the host genome cannot be completely avoided and this has associated safety implications.
Virus-like particlesIn an effort to overcome potential safety concerns associated with traditional viral vectors, researchers have also explored the use of virus-like particles (VLPs) for CRISPR delivery. VLPs are engineered particles; essentially an empty viral capsid, they contain no viral genome, making them non-replicative and non-integrating. They have been used to deliver a range of CRISPR components, including base editors and prime editors.
- Advantages: VLPs have the potential for cell and tissue-specific CRISPR delivery, and are also able to escape endosomes. Unlike real viral vectors, VLPs do not carry the associated safety concerns, such as integration. Additionally, they deliver CRISPR components in a transient fashion, which reduces the possibility of long-term expression and, therefore, off-target editing.
- Disadvantages: The clinical translation of VLPs for CRISPR delivery has been hindered by manufacturing challenges, and further research is needed to streamline and optimize their production at scale. There are also some issues with the stability of VLPs, as well as their cargo size limitations.
In an effort to overcome potential safety concerns associated with traditional viral vectors, researchers have also explored the use of virus-like particles (VLPs) for CRISPR delivery. VLPs are engineered particles; essentially an empty viral capsid, they contain no viral genome, making them non-replicative and non-integrating. They have been used to deliver a range of CRISPR components, including base editors and prime editors.
- Advantages: VLPs have the potential for cell and tissue-specific CRISPR delivery, and are also able to escape endosomes. Unlike real viral vectors, VLPs do not carry the associated safety concerns, such as integration. Additionally, they deliver CRISPR components in a transient fashion, which reduces the possibility of long-term expression and, therefore, off-target editing.
- Disadvantages: The clinical translation of VLPs for CRISPR delivery has been hindered by manufacturing challenges, and further research is needed to streamline and optimize their production at scale. There are also some issues with the stability of VLPs, as well as their cargo size limitations.
Non-viral CRISPR delivery methodsNon-viral methods for CRISPR-Cas9 delivery (nano delivery) include lipid nanoparticles, extracellular vesicles, lipoplexes and polyplexes, inorganic nanoparticles, cell-penetrating peptides, and several other methods.
Non-viral methods for CRISPR-Cas9 delivery (nano delivery) include lipid nanoparticles, extracellular vesicles, lipoplexes and polyplexes, inorganic nanoparticles, cell-penetrating peptides, and several other methods.
Lipid nanoparticles and extracellular vesiclesOne strategy to deliver unstable, anionic nucleic acids into cells and protect them from protease degradation is by encapsulating the cargo in lipids. Lipid nanoparticles (LNPs) are synthetic nanoparticles primarily made up of ionizable lipids, while extracellular vesicles (EVs) are membrane-derived lipid envelopes released naturally from cells that contain bioactive molecules. Both can be given a payload of CRISPR cargo to deliver into cells. LNPs in particular are popular for in vivo CRISPR delivery; they were widely adopted during the COVID-19 pandemic to deliver mRNA payloads in vaccines.
- Advantages: LNPs and EVs have minimal safety and immunogenicity concerns due to lack of viral components, and in the case of EVs, they are derived from human cells. All three types of CRISPR cargo can be delivered using these methods. The last several years have seen significant progress in the development of organ-targeted LNPs. For example, selective organ targeting (SORT) nanoparticles are engineered LNPs attached to a SORT molecule, making them targeted to specific cell types within lung, spleen and liver tissues.
- Disadvantages: A key issue associated with LNPs is the internal cellular processes that occur after delivery. They are usually encased by endosomes, and must ‘escape’ to avoid being directed into the lysosomal pathway and degraded, as well as gain access to the nucleus of the cell. In contrast, EVs have strong potential for tissue-homing but their inherent complexity and heterogeneity have so far hindered their clinical translation.
One strategy to deliver unstable, anionic nucleic acids into cells and protect them from protease degradation is by encapsulating the cargo in lipids. Lipid nanoparticles (LNPs) are synthetic nanoparticles primarily made up of ionizable lipids, while extracellular vesicles (EVs) are membrane-derived lipid envelopes released naturally from cells that contain bioactive molecules. Both can be given a payload of CRISPR cargo to deliver into cells. LNPs in particular are popular for in vivo CRISPR delivery; they were widely adopted during the COVID-19 pandemic to deliver mRNA payloads in vaccines.
- Advantages: LNPs and EVs have minimal safety and immunogenicity concerns due to lack of viral components, and in the case of EVs, they are derived from human cells. All three types of CRISPR cargo can be delivered using these methods. The last several years have seen significant progress in the development of organ-targeted LNPs. For example, selective organ targeting (SORT) nanoparticles are engineered LNPs attached to a SORT molecule, making them targeted to specific cell types within lung, spleen and liver tissues.
- Disadvantages: A key issue associated with LNPs is the internal cellular processes that occur after delivery. They are usually encased by endosomes, and must ‘escape’ to avoid being directed into the lysosomal pathway and degraded, as well as gain access to the nucleus of the cell. In contrast, EVs have strong potential for tissue-homing but their inherent complexity and heterogeneity have so far hindered their clinical translation.
Lipoplexes and polyplexesAnother method for allowing nucleic acids to gain entry into cells is by complexing them with cationic lipids (lipoplexes) or cationic polymers (polyplexes). Although less popular than LNPs, these delivery vehicles are still being actively investigated. For a more detailed breakdown, you can read this review of gene delivery by lipoplexes and polyplexes.
- Advantages: Similar to other non-viral methods, there is less chance of associated severe immune responses when utilizing lipoplexes and polyplexes.
- Disadvantages: As described for liposomes, lipoplexes and polyplexes must escape from endosomes and gain entry to the nucleus of the cell. Various reagents have different transfection efficiencies, with some being better suited for use with certain types of cells.
Another method for allowing nucleic acids to gain entry into cells is by complexing them with cationic lipids (lipoplexes) or cationic polymers (polyplexes). Although less popular than LNPs, these delivery vehicles are still being actively investigated. For a more detailed breakdown, you can read this review of gene delivery by lipoplexes and polyplexes.
- Advantages: Similar to other non-viral methods, there is less chance of associated severe immune responses when utilizing lipoplexes and polyplexes.
- Disadvantages: As described for liposomes, lipoplexes and polyplexes must escape from endosomes and gain entry to the nucleus of the cell. Various reagents have different transfection efficiencies, with some being better suited for use with certain types of cells.
Other nanoparticlesOther types of nanoparticles (NPs) for CRISPR-Cas9 delivery are also being investigated for their potential in CRISPR delivery. They are made up of inorganic materials, including gold (AuNPs), silica, and magnetic particles.
- Advantages: NPs can deliver all three types of CRISPR cargo into cells. While each type of NP will have varying qualities, they generally have high stability and efficiency, relatively low cost, reduced off-target effects, high loading capacity, and lack of immune response and mutagenesis. They are an appealing vehicle for in vitro and in vivo studies.
- Disadvantages: Some NPs tend to aggregate in a physical medium. AuNPs have a key disadvantage of being toxic at high concentrations, and since they are relatively new methods, there are some concerns regarding the long-term toxicity and accumulation of other NPs, such as magnetic NPs.
Other types of nanoparticles (NPs) for CRISPR-Cas9 delivery are also being investigated for their potential in CRISPR delivery. They are made up of inorganic materials, including gold (AuNPs), silica, and magnetic particles.
- Advantages: NPs can deliver all three types of CRISPR cargo into cells. While each type of NP will have varying qualities, they generally have high stability and efficiency, relatively low cost, reduced off-target effects, high loading capacity, and lack of immune response and mutagenesis. They are an appealing vehicle for in vitro and in vivo studies.
- Disadvantages: Some NPs tend to aggregate in a physical medium. AuNPs have a key disadvantage of being toxic at high concentrations, and since they are relatively new methods, there are some concerns regarding the long-term toxicity and accumulation of other NPs, such as magnetic NPs.
Cell-penetrating peptidesAnother non-viral CRISPR delivery method is cell-penetrating peptides (CPPs). CPPs are short, positively charged peptides that facilitate the uptake of molecules by the cell. They are usually conjugated with the CRISPR cargo via covalent bonds.
- Advantages: CPPs can be used for both in vitro and ex vivo CRISPR studies. They can also be used for many different types of cells, including primary human T cells, and can achieve high genome editing efficiencies. There is potential for CPPs to be modified to target specific cell or tissue types.
- Disadvantages: CPPs do not provide the cargo protection that encapsulation techniques like LNPs do. Although recent research has demonstrated their use in animal models, it is unclear if they can be successfully translated to in vivo genome editing in humans.
Another non-viral CRISPR delivery method is cell-penetrating peptides (CPPs). CPPs are short, positively charged peptides that facilitate the uptake of molecules by the cell. They are usually conjugated with the CRISPR cargo via covalent bonds.
- Advantages: CPPs can be used for both in vitro and ex vivo CRISPR studies. They can also be used for many different types of cells, including primary human T cells, and can achieve high genome editing efficiencies. There is potential for CPPs to be modified to target specific cell or tissue types.
- Disadvantages: CPPs do not provide the cargo protection that encapsulation techniques like LNPs do. Although recent research has demonstrated their use in animal models, it is unclear if they can be successfully translated to in vivo genome editing in humans.
Physical deliveryPhysical CRISPR delivery methods include techniques such as electroporation, microinjection, and microfluidics. These delivery methods are typically only suitable for editing in ex vivo cell therapies and in vitro studies. Electroporation is the most common physical CRISPR delivery method and recent technological advances have made it an attractive avenue for the development of gene-edited cell therapies.
Physical CRISPR delivery methods include techniques such as electroporation, microinjection, and microfluidics. These delivery methods are typically only suitable for editing in ex vivo cell therapies and in vitro studies. Electroporation is the most common physical CRISPR delivery method and recent technological advances have made it an attractive avenue for the development of gene-edited cell therapies.
ElectroporationThis technique involves opening transient pores in the membrane of cells via high-voltage electrical currents, allowing CRISPR cargo to gain entry. Electroporation also includes nucleofection, which is a more specialized method that delivers the cargo to the nuclei of cells.
- Advantages: Electroporation can be used for many different cell types, including those that are otherwise difficult to transfect. It can also deliver all three types of CRISPR cargo of any size, and is appropriate for both in vitro and ex vivo studies. This method of CRISPR delivery also avoids the use of viral vectors, which can be preferable for clinical applications.
- Disadvantages: Historically, electroporation has not always been suitable for every experiment or cell type. Mammalian cells, for example, are sensitive to electrical currents and many electroporation methods can change the cell state and compromise cell viability. However, Maxcyte’s scalable, cGMP compliant electroporation technology has enabled the successful CRISPR editing of primary human cells, including T cells, without reducing cell viability. This makes it an attractive CRISPR delivery method for the development of cell therapies.
This technique involves opening transient pores in the membrane of cells via high-voltage electrical currents, allowing CRISPR cargo to gain entry. Electroporation also includes nucleofection, which is a more specialized method that delivers the cargo to the nuclei of cells.
- Advantages: Electroporation can be used for many different cell types, including those that are otherwise difficult to transfect. It can also deliver all three types of CRISPR cargo of any size, and is appropriate for both in vitro and ex vivo studies. This method of CRISPR delivery also avoids the use of viral vectors, which can be preferable for clinical applications.
- Disadvantages: Historically, electroporation has not always been suitable for every experiment or cell type. Mammalian cells, for example, are sensitive to electrical currents and many electroporation methods can change the cell state and compromise cell viability. However, Maxcyte’s scalable, cGMP compliant electroporation technology has enabled the successful CRISPR editing of primary human cells, including T cells, without reducing cell viability. This makes it an attractive CRISPR delivery method for the development of cell therapies.
MicrofluidicsThis less-common CRISPR delivery method involves using microscopic channels to manipulate fluids, making the cell membrane transiently permeable so that cargo can be delivered into cells.
- Advantages: This technique is gentle on cells, and has been demonstrated to be effective when editing primary human immune cells, such as T cells and natural killer cells.
- Disadvantages: Microfluidics has not yet been widely adopted for CRISPR delivery. Many microfluidic chips can only accommodate relatively small numbers of cells, as the arrays of the chip can become clogged with cells.
To learn more, you can check out our interview with Ryan Pawell, CEO of Indee Labs, who discusses µVS and his goal of providing scalable and affordable CRISPR-edited T-cells for immunotherapy.
This less-common CRISPR delivery method involves using microscopic channels to manipulate fluids, making the cell membrane transiently permeable so that cargo can be delivered into cells.
- Advantages: This technique is gentle on cells, and has been demonstrated to be effective when editing primary human immune cells, such as T cells and natural killer cells.
- Disadvantages: Microfluidics has not yet been widely adopted for CRISPR delivery. Many microfluidic chips can only accommodate relatively small numbers of cells, as the arrays of the chip can become clogged with cells.
To learn more, you can check out our interview with Ryan Pawell, CEO of Indee Labs, who discusses µVS and his goal of providing scalable and affordable CRISPR-edited T-cells for immunotherapy.
Ryan Pawell Talks About Indee Labs’ CRISPR Delivery Technology for Affordable Immunotherapies
Ryan Pawell, CEO of Indee Labs, talks about his company’s microfluidic vortex shedding technology for CRISPR delivery in cells. Their mission is to provide a scalable non-viral delivery platform for affordable T-cell immunotherapies for patients.

MicroinjectionMicroinjection delivers CRISPR cargo into individual cells by piercing the membrane with a microscopic needle.
- Advantages: This CRISPR delivery method can deliver cargo of any molecular weight, at known quantities, to either the cytoplasm or nucleus of the cell. It is most often used for delivery of nucleic acid cargo, and has high efficiency. When used to deliver RNA cargo, it will have restricted duration within the cells, typically reducing off-target effects.
- Disadvantages: This method can be a challenging and laborious process. It is limited to animal models and the modification of cells ex vivo, and is not suitable for in vivo studies.
Microinjection delivers CRISPR cargo into individual cells by piercing the membrane with a microscopic needle.
- Advantages: This CRISPR delivery method can deliver cargo of any molecular weight, at known quantities, to either the cytoplasm or nucleus of the cell. It is most often used for delivery of nucleic acid cargo, and has high efficiency. When used to deliver RNA cargo, it will have restricted duration within the cells, typically reducing off-target effects.
- Disadvantages: This method can be a challenging and laborious process. It is limited to animal models and the modification of cells ex vivo, and is not suitable for in vivo studies.
The current state of play in CRISPR delivery
There has been exciting progress in CRISPR delivery systems in recent years, particularly in the improvement of earlier techniques. Researchers continue to address the key challenges in the CRISPR delivery field; generally speaking, the current focus is on creating safer and more efficient delivery methods for CRISPR cell and gene therapies.
AAV vectors are still a mainstay in the clinical CRISPR delivery field, and as we mentioned, they have already been approved by the FDA. Scientists continue to explore the possibilities of engineering AAV capsids to help these vehicles evade the immune system, tailor their tropism, and to enhance their transduction efficiency. AAVs make an attractive CRISPR delivery vehicle for smaller Cas nucleases with high-fidelity gene editing activity, such as Synthego’s hfCas12Max - particularly in the development of in vivo CRISPR therapies. For a detailed history of the use of AAVs as gene delivery vectors and current efforts to maximize their potential, you can read this recent review.

eSpOT-ON is Synthego’s engineered high-fidelity Cas9 variant, designed for therapeutic gene editing. Delivered in mRNA format, eSpOT-ON provides superior on-target activity, low off-target effects, and a reduced risk of chromosomal translocations. Validated in preclinical studies, eSpOT-ON nuclease mRNA and gRNA are ready to support your next breakthrough in cell and gene therapy development.
Learn more about using eSpOT-ON nuclease mRNA.
There has also been increased interest in the use of VLPs and related vehicles for therapeutic CRISPR delivery. In a key development, CRISPR pioneer Jennifer Doudna recently published a study in Nature Biotechnology describing enveloped delivery vehicles (EDVs), a derivative of VLP technology. Using retroviral VLP assembly of mutant vesicular stomatitis virus (VSVGmut) particles, Doudna and her colleagues attached antibody-derived single-chain variable fragments (scFVs) to the outside of the particles, which allowed the Cas9 RNP complexes inside to be delivered to target cells. This technique relies on well-studied interactions between antibodies and antigens to deliver the cargo to specific cell populations. The study demonstrated that T cells could be targeted with high specificity, engineering CAR-T cells in vivo - a remarkable feat for CRISPR delivery.
LNPs also continue to be developed and refined for their safety and tissue-targeting capabilities. In a key development for the CRISPR field, LNPs were used to deliver the world’s first personalized gene therapy to a baby with an ultra-rare genetic disorder. Recent research has also demonstrated the use of LNPs to deliver Synthego’s engineered, high-fidelity eSPOT-ON nuclease in vivo to edit the PCSK9 gene in the mouse liver, an important proof-of-concept study in the treatment of hypercholesterolemia. Similarly, LNPs were used to deliver our hfCas12Max nuclease - an engineered, high fidelity Cas12a variant - to the liver of mice to target the transthyretin (Ttr) gene, achieving robust editing efficiencies.
These versatile CRISPR delivery vehicles have been used to deliver all types of cargo, as well as several different CRISPR systems, such as base editors. Recent studies have demonstrated that they can be targeted to the lungs, muscle tissue, and brain. Importantly, they have been shown to successfully co-deliver Cas9 mRNA, sgRNA, and a single-stranded DNA template for repair via HDR, making them ideal for gene correction and insertion applications, including for the treatment of cystic fibrosis in an animal model.
As we discussed earlier, recent improvements in electroporation technology have enabled this CRISPR delivery technique to be used in the development of edited cell therapies. Maxcyte’s ExPERT electroporation platform allows researchers to deliver diverse CRISPR cargo into a broad range of cell types with high reproducibility and without compromising cell viability - a major regulatory concern in this field. This is particularly relevant when performing editing experiments in primary and stem cells, which are often difficult to edit; electroporation has been demonstrated to successfully deliver hfCas12Max to primary human CD3+ T cells for targeting of the TRAC locus, with high cell viability.
One of the more nascent CRISPR delivery methods is DNA origami, in which long, single-stranded DNA is folded to create a nanostructure. These compact vehicles can deliver cargos of several kilobases, including DNA donor templates for gene correction and insertions. Importantly, they have been demonstrated to successfully deliver CRISPR components to the nuclei of cells. For some exciting developments in targeted delivery of CRISPR, you can read our interview with Adre Watson, CEO and co-founder of Ligandal.
Improving Targeted Delivery to Enhance Personalized Medicine (with Podcast)
In this podcast/article combo, we sat down with CEO and co-founder of Ligandal Andre Watson to learn more about how his company is improving the payload delivery of gene therapy, making it more accurate and efficient, to make new gene therapy techniques a reality for everyday people.

We hope this article gave you an understanding of all the different CRISPR delivery methods that are available and the advantages and challenges associated with each one, so that you can choose the best method for your CRISPR experiments. While a universal method for CRISPR delivery remains elusive, there will undoubtedly be more breakthroughs and developments in this area in the near future.
Interested in more CRISPR-related articles? Check out our Resources section for helpful assets. For more information on how Synthego can help your CRISPR research, you can schedule a free assessment and speak with one of our genome engineering experts now.