Dr. Mark DeWitt Ph.D., Associate Director at Mammoth Biosciences, and Dr. Don Kohn M.D., distinguished professor and Director of the UCLA Human Gene and Cell Therapy Program and CIRM grantee, were destined to work together to find a cure for sickle cell anemia.
In this interview, Dr. DeWitt and Dr. Kohn discussed how each of their sickle cell anemia projects started and converged into their collaborative clinical trial to cure the disease. Leading the discussion, Rebecca Roberts Ph.D. and Synthego’s Head of Science, Kevin Holden Ph.D, you will learn the importance of developing a robust delivery system for clinical applications and how critical it is to have a single supplier of CRISPR reagents from early discovery to clinical studies when there are supply chain issues.
Sickle cell anemia is a red blood cell disorder that affects hemoglobin, the component of the blood cell that carries oxygen in the blood. In this disorder, part of the genetic sequence that codes for hemoglobin contains a mutation resulting in distorting the red blood cell shape to take a sickle, or crescent moon, shape. Utilizing CRISPR to correct the mutations, various cell and gene therapies are hitting the market to cure the disease, like the one Dr. DeWitt and Dr. Kohn developed.
The convergence of sickle cell anemia projects progressed the therapy from the preclinical state to IND submission
Rebecca: Please give us a brief introduction as to your current title and role.
Mark:
I'm an associate director at Mammoth Biosciences, a gene editing company in the Bay Area. It’s one of several companies that spun out of Jennifer Doudna's Lab at Berkeley over the past 10 years. At Mammoth, we're really focused on developing in vivo-directed gene editing therapies using novel nucleases or in a couple of cases [those we] have licensed from Berkelely. The advantage of our nuclease systems really is in their small size which allows them to be neatly packaged into [adeno-associated viral vectors] AAV or [lipid nanoparticles] LNPs for use.
CRISPR Delivery Methods: Cargo, Vehicles, and Challenges
Efficient CRISPR delivery is essential in cell and gene therapies. In this article, we discuss the many different CRISPR delivery methods, including viral, non-viral, and physical.
Don:
I'm Donald Kohn, a professor at UCLA, University of California Los Angeles. I am a pediatric bone marrow transplant physician by clinical training and I've done research on gene therapies for blood cells for my whole career. My lab does more of the basic work of developing new lentiviral vectors or working out [gene] editing techniques [which] we've also brought [many] to clinical trials. So we've done trials over the years for several primary immune deficiency diseases, most notably [adenosine deaminase deficiency that causes severe combined immunodeficiency], ADA SCID, in addition to trials for sickle cell disease, which is what introduced me to Mark.
Kevin: Can you tell us a little bit about how the sickle cell gene therapy project got started at the Innovative Genomics Institute, who was involved in getting it up and started, and what year you started to work on this?
Mark:
The project was first conceived in the meeting between Jennifer Doudna and the eventual PI of the project, Dr. Mark Walters, in Oakland. From there, they brought in my PI, Jacob Corn, as the gene editing expert on the project, and me, his postdoc, as well as David Martin who's also a hematologist who ran a research lab in Oakland [at the time]. This [was] the core group for the first phase, sort of early pre-preclinical phase, of the project that was formed in 2014. [In this phase] an undergrad and I were doing the gene editing work with David and his staff scientist, Wendy Magis, to get the basic protocol [established] in 2014, 2015. [We then] published a paper around the same time that Don joined the team in 2016.
Kevin: Can you walk us through the gene editing strategy? For example, what are you targeting, what nuclease are you using, how did you assess the safety and the profile of the gene edit?
Mark:
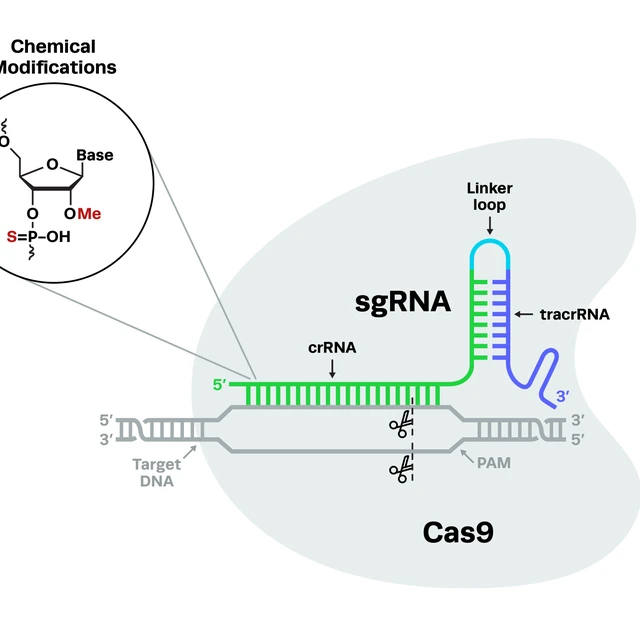
The cells are harvested from mobilized peripheral blood. We then isolate the stem and progenitor CD34 cells using a CliniMACS device. These isolated cells are cultured for two days in media that mildly stimulates them to divide but hopefully not too much because you don't want to deplete stem cells by overstimulation. We then electroporate them with our gene editing reagents. It consists of a Cas9 RNP that targets just downstream of the sickle mutation, along with a short single-stranded DNA that encodes a part of the healthy beta-globin sequence without the sickle mutation. Through homology-directed repair, that sequence is spliced into [the genome] a reasonable fraction of the time reverting the sickle mutation back to wild type. You also create a population of indels, which are genetic scars that likely destroy the expression of the gene too. So you have this mixed population [of cells] where we believe that the corrected cells will win out in the end.
So those edited cells are harvested frozen with aliquots taken for QC testing before release, ultimately for clinical use. So in terms of the safety packet for this therapy, the agency had a lot of questions about off-target editing, so we did a very thorough search for off-targets using a combination of GUIDE-seq and bioinformatic searches to confirm an absence of off-target editing. We also looked at translocations between the on and off-target sites. An important [discovery during these QC testing assessments] was that we did have an off-target at one time that was quite significant. We were able to reduce editing at that off-target by adopting a high-fidelity variant of Cas9 that is widely used in the field which resolved the problem for us. Additionally, the tox packet was actually pretty light which consisted of a mouse tox study with edited human cells and was assessed for four months. This meant that the genomic geno-tox package was the core safety package for our IND.
Kevin: Don, can you tell us about how the project transitioned into your lab and what was required to bring it from the preclinical state into an IND submission?
Don:
So we actually started my lab working on gene editing in collaboration with Sangamo in probably about 2010/2011. They approached us about working on HIV, and then we worked a little bit on ADA and then we started working on sickle cell. When CRISPR came along, a graduate student in the lab, Megan Hoban, [applied the technique to understand sickle cell anemia]. By convergent evolution, we wound up with the same guide sequence as Mark and his group were using because it was the best one near the sickle mutation. Like Mark, we started out making our guides by in vitro transcription (IVT). [Around the same time,] we learned about the modified guides from publications. We then ordered the same guides [with modifications] and were really impressed that the editing went up about two-fold using the synthetic guides, and we never looked back.
How Synthetic Modified Guides Changed Everything
Rebecca: How did the use of chemically modified synthetic CRISPR single guide RNAs enhance the progress of your preclinical work there?
Mark:
We were making our RNAs by IVT using New England Biolab kits. Those are obviously unmodified and the quality is very inconsistent. So we actually first started using synthetic guides through a collaboration with Agilent [where] we immediately noticed that the editing results were better when we used synthetic modified guides. [Additionally] the viability was improved and the consistency of the results was also much, much higher. Shortly after we ran out of our stock from Agilent and the collaboration kind of trailed off was when we started using Synthego’s modified guides and became early adopters.
Kevin: How did it really help move the preclinical work forward when you switched to using [Synthego’s synthetic guides]? Was it the availability to get them quickly and not have to make them? Was it the [high editing in] HSCs? What was the driver or main reason [for the switch to synthetic guides]?
Mark:
Being able to get controlled reagents that delivered consistent results and also gave us better editing results, both in terms of the indels as well as the HDR (homology-directed repair). For this project, we're using a short DNA as an HDR donor to install the mutation or install the corrective mutation. So being able to get guides quickly from Synthego at high quality was an enormous boost to our research [where we performed] protocol optimization and ultimately our CMC development in Don's lab. There was really no other game in town besides Synthego [because] the prices were very reasonable, and the turnaround times were fast.
Contact the Experts
Do you have more questions for our GMP or regulatory specialists? Consult our experts to discuss how to streamline your path to the clinic.

Strategy For Clinical Success
Rebecca: Your gene editing strategy is a non-viral approach where you're seeking to directly repair the mutation in the sickle allele. Can you tell us how this strategy differs from other approaches that are currently in the clinic?
Mark:
Both Don's lab and our labs up in Berkeley [were] thinking along very similar lines. We were both looking at either single-stranded DNA or AAV donors. Our belief at the time was that directly correcting the mutation is more penetrant because you get a corrected allele that is 100% as opposed to turning on a compensatory gene like fetal hemoglobin but we weren't sure about epigenetic editing. At the time, we believed epigenetic editing was an editing enhancer of a transcription factor or other more indirect methods that had inherent complexities and risks associated more with biology.
Another sort of core of the belief was from our understanding of the hematology of sickle cell disease wherein sickle red blood cells contain two indels that produce no beta globin at all [resulting in] less efficient maturation of the red blood cells greatly reducing their half-life. So even a small percentage of genetic correction of the red blood cells on either allele would be able to produce a persistent healthy cell that would thus outcompete the remaining disease cells in the circulation. So that was the rationale for pursuing direct correction using CRISPR.
The History of CRISPR Cell and Gene Therapies
CRISPR cell and gene therapies can treat genetic diseases, cancer, and more. This article explores the history of cell and gene therapies, the CRISPR revolution, and the current state of the field.

Supply Chain Challenges Can Impede Progress Of Therapy Development
Kevin: Have you experienced any supply chain issues when it comes to the reagents for your IND-enabling work or for the upcoming trial? How important is it to be able to stick with a single vendor all the way through the process?
Mark:
It's vitally important to advance your project in a timely manner to ensure that your results are comparable across your preclinical and clinical studies. I would be lying if I said I didn't learn that lesson the hard way. I think most of us in this industry have had at least one or two war stories in that department. The main lesson that I've learned and learned from my more experienced colleagues in the CMC field is the importance of maintaining a durable, consistent, collaborative relationship with your suppliers. Ideally, start as early in your discovery phase as possible.
Synthego really stands out because I'm not sure if there are many others that can offer screening scale, research use guides for your lead discovery, medium to large scale RUO guides for your preclinical and pharmacology studies, and ultimately larger scale GLP Tox and clinical grade material for early and hopefully ultimately late phase trials. So, I wish there were more Synthego out there where you could partner with the same company for at least the first half of development [to] ideally the rest of [clinical trials] because I can tell you for a fact that changing suppliers when you're in the clinic is a real challenge.
Don:
I strongly second that lesson learned the hard way. In academics where we try and get a trial going, treat a few patients, and see if it works, we often don't have the budgets to go with a high quality vendor for reagents from the start. If [the trial is] successful, everything has to start again when you move up to the high quality reagent. So starting from the beginning as much as possible with something that could be commercialized really is critical.
CRISPR sgRNA for Successful Gene Editing: Discovery to Clinic
The single guide RNA (sgRNA) is one of the key components of successful CRISPR gene editing. In this blog, we cover all the important aspects of sgRNA and Synthego’s sgRNA products.
Progress Of Curing Sickle Cell Anemia In Clinical Trials
Rebecca: Can you tell us about [your] upcoming clinical trial? When and where will it be taking place, how many patients will you be looking to dose in the first phase, and then the subsequent phases?
Don:
It’s public information and has been funded through the California Institute for Regenerative Medicine (CIRM) composed of three successive grants for the pre-IND work, the IND-enabling work, and now for the clinical trial. We are also co-funded by NHLBIs Cure Sickle Initiative so funding is split between CIRM and NHLBIs Cure Sickle Initiative. [For the clinical trials,] the plan is to treat nine patients [where the] initial cohort of three patients 18 years or older [go through treatment], then a pause to look for safety and efficacy, then the next three patients [will undergo treatment]. If we have success with the first six patients who are 18 years or older, we will move on to an adolescent cohort down to 12 years of age.
Rebecca: On that note, how long will it be before you know if the trial has had a real successful impact on those patients? What are your sort of clinical endpoints?
Don:
Since this is done in the context of a bone marrow transplant, we collect their stem cells, they're edited, and then that's frozen away as the drug product, which is then tested for release. When we know it meets qualifications, the patient comes back in and gets chemotherapy to get rid of the rest of the bone marrow in their body. By giving the right dose, we can eliminate the remaining stem cells and repopulate their hematopoietic system [with our CRISPR-edited cells]. So the first milestone is do their counts, recover, do their hematologic reconstitution. About 21 days to 30 days or so after the transplant, we want to see them making neutrophils, making platelets, and red cells while not needing a transfusion. Also at this milestone, we should be able to see the edited cells in their circulation. Since we are editing stem cells and, although we really care about the red cells containing the active gene, we should be able to see the edit in all their cells.
So even in the first month, once they start making white blood cells from the transplant, we can see if they are edited which is our first glimmer of efficacy. If we see edited cells early on and then over the proceeding three to six month period where they are now making good red cells that won't sickle, we should see them not needing transfusions because the cells have the gene corrected. [We can assess this through blood tests by seeing] them make their own red cells with hemoglobin A, the normal hemoglobin, and a drop in the cell with hemoglobin S, mutant hemoglobin.
For this trial, we are following the patients for two years where the real clinical endpoint is do the patients stop having sickle crises. Patients with sickle cell will have these crises at various intervals monthly where they have severe pain because of the red cells sickling in the little blood vessels. [To assess our clinical endpoint,] we will record the frequency that the patients have crises for the two years before the treatment and then the two years after to hopefully show that we've eliminated the most severe complication of sickle cell for the patient.
CRISPR in the Clinic: Synthego’s Regulatory Experts Discuss Development of Cell and Gene Therapies
The CRISPR gene therapy regulatory space is changing rapidly. In this blog, we’re shining a light on regulatory scientists, the unsung heroes of clinical development.

Advice For Pursuing Careers in CRISPR Cell Therapies
Kevin: You obviously have a lot of experience and learned a lot over the years from gene editing and going through the IND process. Do you have any advice for any researchers out there who are looking to forge a career in CRISPR cell therapies or a related field?
Mark:
I like to work on things that I feel passionate about with the kind of people that make you wanna get up early and stay late in lab not because anyone is making you but because you want to. I can't imagine operating any other way than that. I guess that's one piece of advice. I also think your mentors are really important, particularly early on. Don is an excellent one and there are others. Really take some time to, you know, stop and smell the flowers, and don't worry too much about what your ultimate goals are knowing that it's still early. It is better to just learn, absorb, and work on things that you are passionate about and save the empire-building for the middle or latter parts of your career perhaps.
In terms of what specifically to work on, these new technologies that move beyond double-strand breaks have tremendous promise and have advanced incredibly rapidly. Most of them are CRISPR-based. I think maybe even the next generation will not be CRISPR-based. So I think if you adopt and understand those emerging technologies now early in your career, you will have an advantage over the competition.
Don:
You have to really go all in and love the science and live the science; the biochemistry, the biophysics, the molecular biology, the genetics. You have to know as much as possible so just be curious, read a lot, learn, work hard, all those kinds of good values are what it takes to get there.
Kevin: How important is it for companies that [are in the cell and gene therapy] space to democratize and advance these [gene editing] technologies? How important is it for them to keep pace with the progress of the cell and gene therapy work that's going on in labs like yours?
Don:
I think it is important. [In] 10 years we've gone from proof of principle to third-generation iterations [of CRISPR]. It's explosive and whoever's playing in the space needs to be looking all the time [for the latest advancements].
CRISPR Cell and Gene Therapies: Key Challenges in Clinical Development
Researchers face many hurdles when developing CRISPR therapy products, including the procurement of true GMP reagents, unclear regulatory guidelines, lack of consistency during development, and shortages of qualified experts. Read more about these issues in CRISPR therapy development and strategies to overcome them.

CRISPR’s Positive Impact On Gene Therapies
Rebecca: So you've got heaps of experience taking CRISPR therapies and other therapies from bench to bedside and you started well before CRISPR was available or a popular technology. Can you tell us a little bit about the positive impact of gene therapies in general and how CRISPR specifically has helped to enable this compared to those previous technologies?
Don:
I was just part of a think tank on how we get access to gene therapies for pediatric cancer and rare diseases which is a big problem with the industry. [There has been] disinvestment in pediatric diseases because they're such small markets. I put together a talk on this looking at what gene therapy has done. For example, it has brought eyesight to the blind, the ones for sickle cell disease from Bluebird and from CRISPR Therapeutics help sickle patients stop having sickle crises, the one for spinal muscular atrophy, and the one for metachromatic leukodystrophy; terrible degenerative and neurologic conditions of children are being cured.
So there really are miracles that gene therapy is doing and I don't think I'm being hyperbolic. I think we sometimes get so caught up in the day-to-day that we lose that big picture. I've been in the field when nothing worked and then things work when they were toxic. Now there are cures and there are seven currently approved sorts of cell and gene therapies for rare diseases and many more for cancer. It's an amazing field because it gets to the basic fundamental problem for these diseases of the genes and it fixes them. I think we're just at the very beginning but there are so many possibiliti