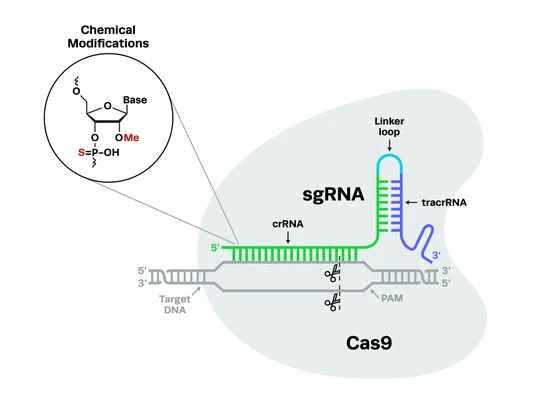
Most researchers familiar with CRISPR technology will have come across the concept of adding chemical modifications to CRISPR guide RNAs (gRNAs). You may have even used chemically modified guides in your research. But unless you’re a chemistry buff, you might still be wondering what these chemical modifications are and what they actually do!
The addition of chemical modifications is crucial, essentially acting as armor for your gRNAs. As such, these modifications can disproportionately affect the outcomes of your CRISPR experiments. Modifications make editing in any cell type more efficient, particularly challenging cells such as primary cells, but they are particularly important for any in vivo CRISPR editing. They are also applicable to any CRISPR system, from CRISPR-Cas9 to base and prime editing.
In this article, we’re demystifying the concept of chemically modifying CRISPR gRNAs. Whether you’re completely new to CRISPR technology, designing guide RNAs for the first time, or creating a CRISPR-based therapy for clinical use, this article will quickly clear up any confusion you have about chemical modifications on CRISPR gRNAs and why you need them.
Chemical Modification of CRISPR gRNA: The Basics
To understand what gRNA chemical modifications are, why they are necessary, and where they are placed, we first need to examine the structure and ‘anatomy’ of the gRNA molecule and a little bit of the history of CRISPR’s use in specific applications.
CRISPR gRNA anatomy and structure
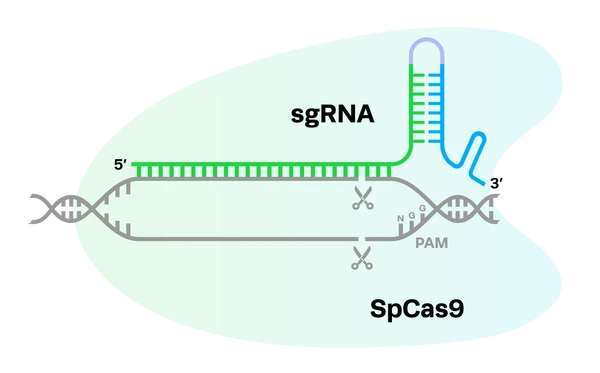
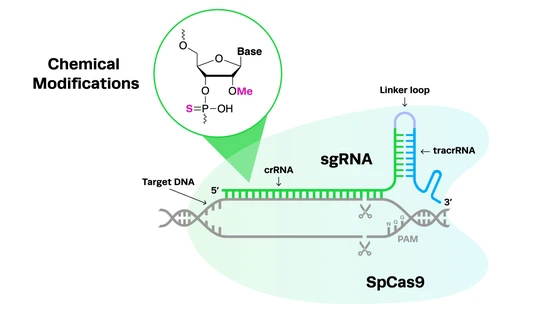
CRISPR-Cas9 systems rely on a two-part gRNA molecule to direct them to the target site. The crispr RNA (crRNA) is complementary to the target site and is 17-20 nucleotides long. The trans-activating RNA (tracrRNA), which acts as a handle for the Cas nuclease, is typically around 65-85 nucleotides. The two parts of the guide can be fused together with a linker loop, forming an RNA chimera known as a single guide RNA (sgRNA).
sgRNA molecules are usually about 100 nucleotides long, with the crRNA at the 5’ end and the tracrRNA at the 3’ end. The seed region refers to 8-10 bases at the 3’ end of the targeting (crRNA) sequence. This area of the molecule plays a crucial role in the binding of the CRISPR complex to the DNA target sequence. Other CRISPR systems use different guide formats, but all of them are RNA-guided.
Now for a little bit of chemistry – this will be important when we discuss specific modifications later on. Like any RNA molecule, the backbone of gRNA* is made up of alternating phosphate (PO) groups and ribose rings, which attach to the nucleic acid bases that make up the sequence. The phosphate groups and sugars are held together by phosphodiester bonds. The ribose rings are 5-carbon sugars (1’ – 5’), with a hydroxyl group (-OH) on each carbon.
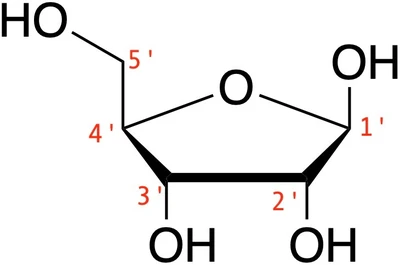
*Because not all researchers or CRISPR applications use the sgRNA format, and because chemical modifications can be added to the individual crRNA and tracrRNA molecules, we will refer to both types of CRISPR guides under the collective term ‘gRNAs’ for the remainder of this article.
Why do CRISPR guides need chemical modifications?The 2012 publication of the original CRISPR-SpCas9 genome engineering system, which demonstrated editing in bacterial cells, was a watershed moment in biology. The simplicity and accuracy of the system led to its rapid adoption by scientists across the globe for a range of different applications.
There was just one major problem: when it came to applying the CRISPR-SpCas9 system in primary human cells, the results weren’t nearly as robust as expected. Editing efficiencies were low, and many cells didn’t survive the editing process, making researchers wonder if CRISPR systems could ever be used to create therapeutic products.
The primary cause of these disappointing results was not the ability of the SpCas9 nuclease, but rather the gRNA molecule that tells SpCas9 where to create double-stranded breaks in the target genome. The first issue is that RNA is a notoriously unstable molecule and is highly prone to degradation. Without protection from enzymes like exonucleases, the gRNA can be degraded before it is able to locate the target sequence, resulting in low editing efficiencies.
Other responses triggered by the innate immune system of primary human cells also present a major problem for CRISPR gRNAs. When infected with a virus, a cell can trigger apoptosis to avoid spreading the infection to its neighbors. Unfortunately, the same mechanism is often triggered in response to foreign strands of gRNA, leading to low yields of edited cells.
It wasn’t until 2015 that Matthew Porteus and his colleagues at Stanford, Ayal Hendel and Rasmus Bak, created a solution to these problems, demonstrating that synthetic sgRNA could be chemically modified to protect it from exonucleases. The authors tested three different types of chemical modifications on the three terminal nucleotides at both the 5’ and 3’ ends of the molecule.
The results? Enhanced CRISPR editing in two clinically relevant cell types - primary human T cells and CD34+ hematopoietic stem and progenitor cells (HSPCs). It’s important to note that this would not have been possible if the authors hadn’t used synthetic sgRNA. Unlike in vitro transcribed (IVT) or plasmid-expressed guide formats, sgRNAs that have been synthesized in the lab are the only type that can be chemically modified.
In the years since this groundbreaking publication, many different types of sgRNA chemical modifications have been tested to examine their effects on the outcome of CRISPR editing. These modifications have played a key role in enabling the development of a variety of genomic medicines, including ex vivo CRISPR cell therapies and in vivo CRISPR gene therapies.
The 2012 publication of the original CRISPR-SpCas9 genome engineering system, which demonstrated editing in bacterial cells, was a watershed moment in biology. The simplicity and accuracy of the system led to its rapid adoption by scientists across the globe for a range of different applications.
There was just one major problem: when it came to applying the CRISPR-SpCas9 system in primary human cells, the results weren’t nearly as robust as expected. Editing efficiencies were low, and many cells didn’t survive the editing process, making researchers wonder if CRISPR systems could ever be used to create therapeutic products.
The primary cause of these disappointing results was not the ability of the SpCas9 nuclease, but rather the gRNA molecule that tells SpCas9 where to create double-stranded breaks in the target genome. The first issue is that RNA is a notoriously unstable molecule and is highly prone to degradation. Without protection from enzymes like exonucleases, the gRNA can be degraded before it is able to locate the target sequence, resulting in low editing efficiencies.
Other responses triggered by the innate immune system of primary human cells also present a major problem for CRISPR gRNAs. When infected with a virus, a cell can trigger apoptosis to avoid spreading the infection to its neighbors. Unfortunately, the same mechanism is often triggered in response to foreign strands of gRNA, leading to low yields of edited cells.
It wasn’t until 2015 that Matthew Porteus and his colleagues at Stanford, Ayal Hendel and Rasmus Bak, created a solution to these problems, demonstrating that synthetic sgRNA could be chemically modified to protect it from exonucleases. The authors tested three different types of chemical modifications on the three terminal nucleotides at both the 5’ and 3’ ends of the molecule.
The results? Enhanced CRISPR editing in two clinically relevant cell types - primary human T cells and CD34+ hematopoietic stem and progenitor cells (HSPCs). It’s important to note that this would not have been possible if the authors hadn’t used synthetic sgRNA. Unlike in vitro transcribed (IVT) or plasmid-expressed guide formats, sgRNAs that have been synthesized in the lab are the only type that can be chemically modified.
In the years since this groundbreaking publication, many different types of sgRNA chemical modifications have been tested to examine their effects on the outcome of CRISPR editing. These modifications have played a key role in enabling the development of a variety of genomic medicines, including ex vivo CRISPR cell therapies and in vivo CRISPR gene therapies.
Synthetic sgRNAs Enable Researchers to Study Viral Infection in Resting Human CD4+ T Cells
Genome editing in resting CD4+ T cells is intrinsically challenging due to limited viability and poor editing efficiency. This historically limits the downstream functional assays, including pathway analyses of these primary T cells. In this case study, developed in conjunction with Lonza, we will learn how using a combination of cell culture conditions, Synthego’s Research sgRNA, and Lonza’s 4D-NucleofectorⓇ enabled Dr. Manuel Albanese and his team to achieve unprecedented knockout editing efficiencies and sustained viability in primary T cells. These studies demonstrated that high-efficiency CRISPR T cell editing can have implications for successfully developing CAR-T cell immunotherapies to treat cancer and viral infections. Dr. Albanese and Dr. Adrian Ruhle conducted this study in Dr. Oliver Keppler’s lab at the Max von Pettenkofer Institute and Gene Center in Munich, Germany.

The importance of location for chemical modificationsChemical modifications can be added to the phosphate groups or ribose sugars that make up the backbone of the sgRNA molecule, or they can be added to the nucleic acid bases. In any case, they are usually made at the 5’ end of the gRNA strand, the 3’ end, or both. Some modifications are made to specific locations on the ribose rings, for example, at the 2’ position of the ribose.
The location of chemical modifications on the gRNA strand is crucial for several reasons. One is that certain areas of the molecule need more protection than others - exonucleases degrade the gRNA from both the 5’ and 3’ ends of the molecule, making these areas particularly vulnerable.
The location of the modifications is also heavily dependent on the nuclease being used. For example, it is common to add chemical modifications to both the 5’ and 3’ ends of the gRNA for SpCas9, while Cas12a will not tolerate any 5’ modifications. Synthego’s high-fidelity Cas12 nuclease, hfCas12Max, functions best with modifications at both ends of the gRNA, but requires slightly different modifications at the 3’ end compared to SpCas9. Synthego’s eSpOT-ON also has its gRNA engineered for use with both its protein and mRNA nuclease formats that features distinct chemical modifications at specific sites to enhance binding affinity and overall editing performance.
Importantly, chemical modifications cannot be made in the seed region of the gRNA, as this may impair the hybridization of gRNA to the target DNA sequence and result in poor editing. The effect of the modifications on the structure of the gRNA molecule is also paramount, as the gRNA needs to retain its A-form helical structure.
Given that the above critical considerations are taken into account, it might surprise you to discover that the specific pattern of the modifications – for example, the exact placement of modifications at the 5’ end – makes no difference to the biological outcomes of editing. Each company that produces synthetic gRNAs will have its own specific placement or pattern of certain chemical modifications on the molecule, but none of these patterns are necessarily better than others.
Chemical modifications can be added to the phosphate groups or ribose sugars that make up the backbone of the sgRNA molecule, or they can be added to the nucleic acid bases. In any case, they are usually made at the 5’ end of the gRNA strand, the 3’ end, or both. Some modifications are made to specific locations on the ribose rings, for example, at the 2’ position of the ribose.
The location of chemical modifications on the gRNA strand is crucial for several reasons. One is that certain areas of the molecule need more protection than others - exonucleases degrade the gRNA from both the 5’ and 3’ ends of the molecule, making these areas particularly vulnerable.
The location of the modifications is also heavily dependent on the nuclease being used. For example, it is common to add chemical modifications to both the 5’ and 3’ ends of the gRNA for SpCas9, while Cas12a will not tolerate any 5’ modifications. Synthego’s high-fidelity Cas12 nuclease, hfCas12Max, functions best with modifications at both ends of the gRNA, but requires slightly different modifications at the 3’ end compared to SpCas9. Synthego’s eSpOT-ON also has its gRNA engineered for use with both its protein and mRNA nuclease formats that features distinct chemical modifications at specific sites to enhance binding affinity and overall editing performance.
Importantly, chemical modifications cannot be made in the seed region of the gRNA, as this may impair the hybridization of gRNA to the target DNA sequence and result in poor editing. The effect of the modifications on the structure of the gRNA molecule is also paramount, as the gRNA needs to retain its A-form helical structure.
Given that the above critical considerations are taken into account, it might surprise you to discover that the specific pattern of the modifications – for example, the exact placement of modifications at the 5’ end – makes no difference to the biological outcomes of editing. Each company that produces synthetic gRNAs will have its own specific placement or pattern of certain chemical modifications on the molecule, but none of these patterns are necessarily better than others.
Types of Chemical Modifications and Their Applications
Several different types of chemical modifications can be applied to CRISPR gRNAs to improve editing efficiencies. While most stabilize the molecule, some chemical modifications have other functions, from reducing off-target editing to amplifying Cas9’s biochemical activity. Other modifications may be used for specific CRISPR derivative technologies, such as prime editing, or to create additional functions beyond the editing process. Let’s explore the various chemical modifications for gRNAs and their applications.
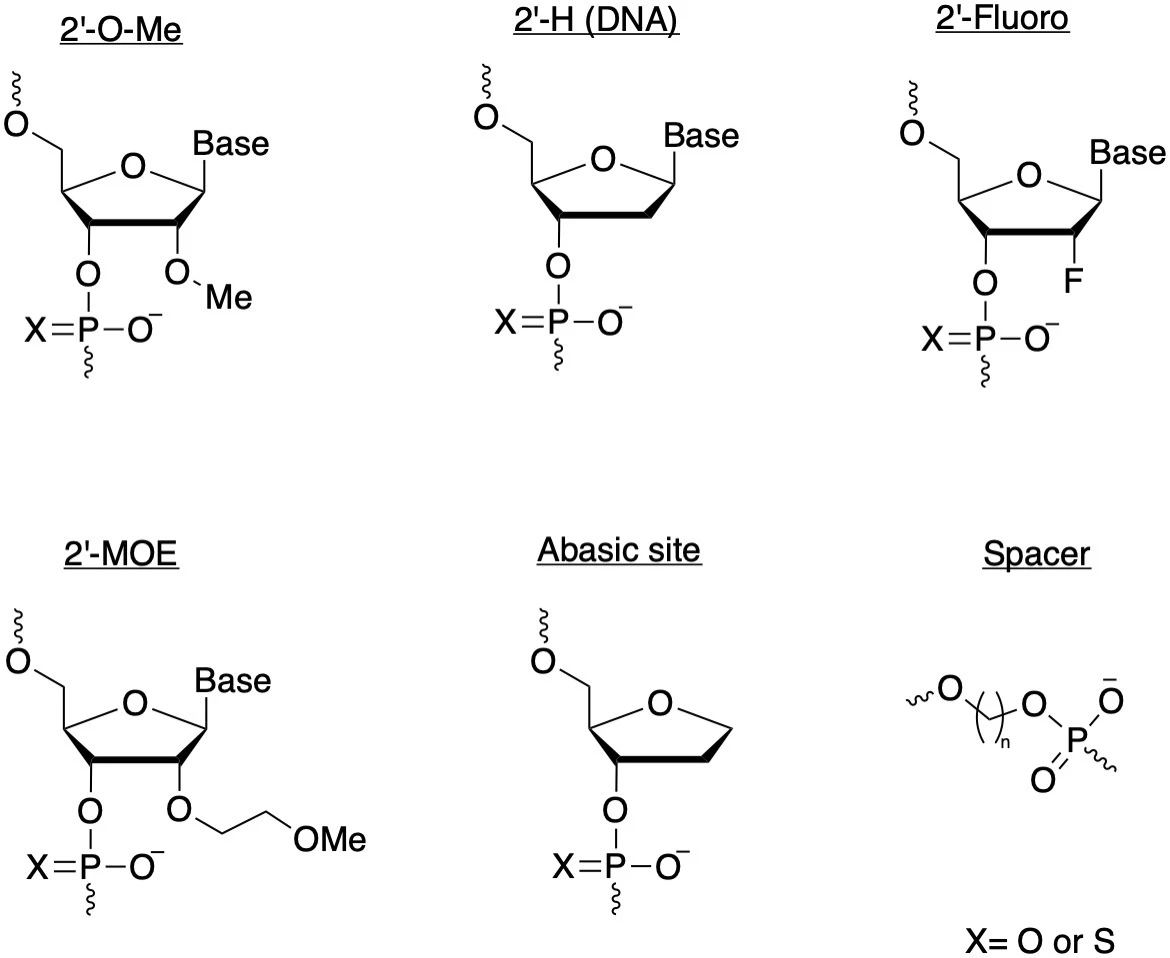
Grow a spine: Backbone modificationsOne of the most widely used ‘families’ of gRNA chemical modifications are those added to the backbone structure of the sgRNA molecule. 2’-O-methylation (2’-O-Me) is a backbone modification in which a methyl group (-CH3) is added to the 2′ hydroxyl (–OH) on a ribose.
2’-O-Me is the most common naturally occurring post-transcriptional RNA modification, protecting the molecule from nucleases and increasing its stability, among other things. 2’-O-Me modified sgRNAs have been shown to increase the specificity of Cas12a systems, but they are also commonly used for SpCas9 and indeed, most CRISPR systems.
Another common type of gRNA backbone modification is the addition of phosphorothioate (PS) bonds. On the phosphate region of the gRNA backbone, a PS bond substitutes the non-bridging oxygen molecule for a sulfur, making the link resistant to nucleases.
2’-O-Me and PS modifications are often used together, in which case they are referred to as 2′-O-methyl 3′ phosphorothioate (MS). This was demonstrated in the 2015 paper we discussed earlier, providing the gRNA molecule with more stability than either modification alone. 2′-O-methyl-3′-phosphonoacetate (2′-O-methyl-3′-PACE, or simply MP) modifications are another variation that has been shown to reduce off-target editing of CRISPR systems while maintaining on-target editing.
Because they increase stability and reduce off-target editing, 2’-O-Me and PS bonds are added to Synthego’s standard gRNAs at both the 5’ and 3’ ends of the molecule. In a 2021 study co-authored by Synthego, researchers from New York University used Synthego’s modified guides to perform efficient RNA knockdown of multiple targets in primary human T cells, which have historically been challenging to edit.
The ribose ring can also be modified by substituting the 2’ hydroxyl group for a fluorine (2’ Fluoro). Locked nucleic acids (LNAs) are a less common backbone modification in which the 2’ oxygen of the ribose ring is linked to the 4’ carbon via a methylene bridge. These modifications give further structural stability to the gRNA molecule and resist degradation by exonucleases.
One of the most widely used ‘families’ of gRNA chemical modifications are those added to the backbone structure of the sgRNA molecule. 2’-O-methylation (2’-O-Me) is a backbone modification in which a methyl group (-CH3) is added to the 2′ hydroxyl (–OH) on a ribose.
2’-O-Me is the most common naturally occurring post-transcriptional RNA modification, protecting the molecule from nucleases and increasing its stability, among other things. 2’-O-Me modified sgRNAs have been shown to increase the specificity of Cas12a systems, but they are also commonly used for SpCas9 and indeed, most CRISPR systems.
Another common type of gRNA backbone modification is the addition of phosphorothioate (PS) bonds. On the phosphate region of the gRNA backbone, a PS bond substitutes the non-bridging oxygen molecule for a sulfur, making the link resistant to nucleases.
2’-O-Me and PS modifications are often used together, in which case they are referred to as 2′-O-methyl 3′ phosphorothioate (MS). This was demonstrated in the 2015 paper we discussed earlier, providing the gRNA molecule with more stability than either modification alone. 2′-O-methyl-3′-phosphonoacetate (2′-O-methyl-3′-PACE, or simply MP) modifications are another variation that has been shown to reduce off-target editing of CRISPR systems while maintaining on-target editing.
Because they increase stability and reduce off-target editing, 2’-O-Me and PS bonds are added to Synthego’s standard gRNAs at both the 5’ and 3’ ends of the molecule. In a 2021 study co-authored by Synthego, researchers from New York University used Synthego’s modified guides to perform efficient RNA knockdown of multiple targets in primary human T cells, which have historically been challenging to edit.
The ribose ring can also be modified by substituting the 2’ hydroxyl group for a fluorine (2’ Fluoro). Locked nucleic acids (LNAs) are a less common backbone modification in which the 2’ oxygen of the ribose ring is linked to the 4’ carbon via a methylene bridge. These modifications give further structural stability to the gRNA molecule and resist degradation by exonucleases.
Not so basic: Base modificationsIn addition to backbone modifications, the bases of the gRNA can also be modified. As we mentioned earlier, RNA is not the most stable molecule, and most chemical modifications aim to make gRNAs more stable. Another approach to this problem is to substitute part of the RNA for a more stable alternative: DNA. Provided they are only in specific regions – for example, not in the seed region of the molecule – DNA bases can increase on-target editing of Cas9.
It's also possible to remove a base entirely from the ribose ring and instead add a spacer or leave an abasic site. Abasic sites in guides are particularly relevant in prime editing because they prevent the unintended integration of the scaffold of the pegRNA (the type of guide used for prime editing).
In addition to backbone modifications, the bases of the gRNA can also be modified. As we mentioned earlier, RNA is not the most stable molecule, and most chemical modifications aim to make gRNAs more stable. Another approach to this problem is to substitute part of the RNA for a more stable alternative: DNA. Provided they are only in specific regions – for example, not in the seed region of the molecule – DNA bases can increase on-target editing of Cas9.
It's also possible to remove a base entirely from the ribose ring and instead add a spacer or leave an abasic site. Abasic sites in guides are particularly relevant in prime editing because they prevent the unintended integration of the scaffold of the pegRNA (the type of guide used for prime editing).
Adding complexity: Functional groups
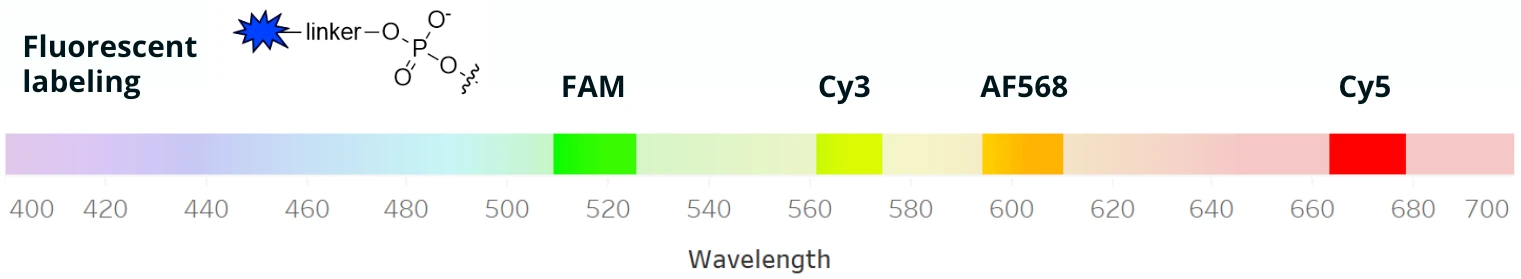
The addition of functional groups to gRNAs via linkers is another type of chemical modification that enables specific applications. Fluorescent labels, such as FAM, Cy3, AF568, or Cy5, are often added to gRNAs to allow the visualization of the molecule for mechanistic studies and other downstream experiments.
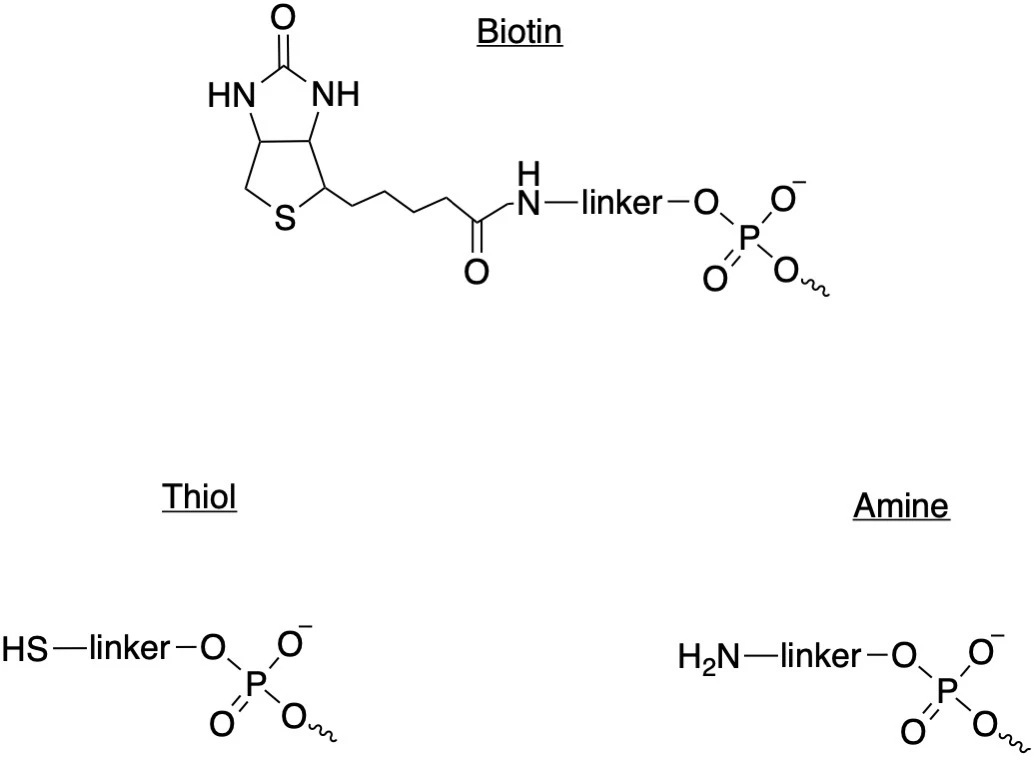
Some functional groups are used for very specific applications; the addition of a 3’ biotin has been used to identify proteins in human cells that can bind to gRNAs and can therefore affect its stability and availability for editing. Other functional groups, such as thiols or amines, are protective or reactive compounds.
Like backbone modifications, more than one functional group can be added to gRNAs for different purposes. For example, Synthego collaborated on a study that used Cas13d to target the SARS-CoV-2 virus in vitro and reduce viral titer, adding both a fluorescent Cy5 label for visualization and a protective thiol group to the crRNA.
By adding a functional group to sgRNAs, Synthego has also generated a CRISPR editing system that can be spatially and temporally controlled to improve its safety profile: CRISPRon and CRISPRoff. A coumarin linker can be cleaved with either blue or UV light; the addition of this functional group to the sgRNA means that editing can be induced using blue light (CRISPRon) and switched off using UV light (CRISPRoff).
That’s heavy: Highly modified guidesThe addition of chemical modifications is particularly important for in vivo applications of CRISPR technology, and in many cases, it requires what we call heavily modified, or highly modified gRNAs. Consider the perilous journey of a gRNA packaged in a lipid nanoparticle (LNP) when it’s delivered in vivo to a human patient, compared to being added to a flask of cells in vitro.
First, it needs to travel to the specific location or organ where editing is required. Once it arrives at the target site, the gRNA and Cas nuclease must penetrate the nucleus of the cell. This process of getting to the site of editing takes time – long enough for an RNA molecule to degrade. But the gRNA must also resist an onslaught of attacks by exonucleases and intra-cellular immune responses.
Highly modified guides have the same modifications we discussed above, but more of them, endowing them with increased structural stability and resistance to exonucleases and making them less likely to degrade in vivo. They are also less likely to elicit innate immune responses.
Synthego’s standard SpCas9 gRNAs are often modified to contain 2’-O-Me and PS bonds at both the 5’ and 3’ ends of the molecule. If the intended application is in vivo gene therapy, we synthesize highly modified gRNAs containing more of these same modifications at more locations on the molecule.
In a notable example, Synthego provided highly modified gRNAs targeting the PCSK9 gene to researchers from the University of Zurich, who generated an adenine base editing (ABE) therapy for familial hypercholesterolemia. As part of their preclinical research, the authors tested their ABE system in both a mouse model and a large animal model of the disease. Synthego’s highly modified gRNAs enabled high-efficiency editing in vivo in the liver of macaques, making it the first study to demonstrate ABE in vivo in a large animal model.
Taking it a step further, fully modified gRNAs have chemical modifications at as many positions as possible to further protect and stabilize the molecule for in vivo applications. For example, they may have modifications at every position on the ribose ring, leaving no -OH groups. A 2018 study from the University of Massachusetts pushed the boundaries of the number of chemical modifications that could be tolerated by the SpCas9 system, demonstrating that several highly modified and fully modified guides were more potent than unmodified versions.
The addition of chemical modifications is particularly important for in vivo applications of CRISPR technology, and in many cases, it requires what we call heavily modified, or highly modified gRNAs. Consider the perilous journey of a gRNA packaged in a lipid nanoparticle (LNP) when it’s delivered in vivo to a human patient, compared to being added to a flask of cells in vitro.
First, it needs to travel to the specific location or organ where editing is required. Once it arrives at the target site, the gRNA and Cas nuclease must penetrate the nucleus of the cell. This process of getting to the site of editing takes time – long enough for an RNA molecule to degrade. But the gRNA must also resist an onslaught of attacks by exonucleases and intra-cellular immune responses.
Highly modified guides have the same modifications we discussed above, but more of them, endowing them with increased structural stability and resistance to exonucleases and making them less likely to degrade in vivo. They are also less likely to elicit innate immune responses.
Synthego’s standard SpCas9 gRNAs are often modified to contain 2’-O-Me and PS bonds at both the 5’ and 3’ ends of the molecule. If the intended application is in vivo gene therapy, we synthesize highly modified gRNAs containing more of these same modifications at more locations on the molecule.
In a notable example, Synthego provided highly modified gRNAs targeting the PCSK9 gene to researchers from the University of Zurich, who generated an adenine base editing (ABE) therapy for familial hypercholesterolemia. As part of their preclinical research, the authors tested their ABE system in both a mouse model and a large animal model of the disease. Synthego’s highly modified gRNAs enabled high-efficiency editing in vivo in the liver of macaques, making it the first study to demonstrate ABE in vivo in a large animal model.
Taking it a step further, fully modified gRNAs have chemical modifications at as many positions as possible to further protect and stabilize the molecule for in vivo applications. For example, they may have modifications at every position on the ribose ring, leaving no -OH groups. A 2018 study from the University of Massachusetts pushed the boundaries of the number of chemical modifications that could be tolerated by the SpCas9 system, demonstrating that several highly modified and fully modified guides were more potent than unmodified versions.
Ensure Clinical Success with Synthego’s Synthetic Chemically Modified sgRNAs
Synthego’s mission is to accelerate the development of life-saving CRISPR cell and gene therapies by providing best-in-class synthetic gRNAs. We provide chemically modified gRNAs for our own nucleases SpCas9, hfCas12Max, and eSpOT-ON (both recombinant protein and mRNA formats), as well as guides for a variety of other CRISPR nucleases, including Cas13d, base editors, and prime editors.
As we discussed earlier, we often add standard modifications to our SpCas9 guides to stabilize the molecules, protect them from degradation by exonucleases, and reduce intracellular immune responses. However, we can also provide highly modified guides - no matter the application, we can provide the right guides to ensure your editing experiments are successful.
The addition of chemical modifications needs to be considered from the earliest days of your CRISPR journey, particularly when you intend to take a CRISPR therapy from bench to bedside. In addition, bottlenecks in the supply chain for clinical-grade reagents are one of the key challenges experienced by sponsors of CRISPR therapies, slowing clinical timeframes.
This makes it crucial to select a gRNA supplier that can not only provide the modifications you need but also produce the modified guides at scale when you begin progressing through clinical trials. Synthego’s state-of-the-art good manufacturing practice (GMP) production facility ensures we can provide the highest-purity chemically modified synthetic gRNAs to our clinical customers while manufacturing at the scales needed to secure the supply chain.
We hope this blog helped you wrap your head around the complexity of adding chemical modifications to CRISPR gRNAs! Want more CRISPR news, resources, and educational content? Don’t forget to subscribe to The Bench Blog, and check out the CRISPR Cuts Podcast.
Enabling GMP Production of sgRNA for CRISPR-based Cell and Gene Therapies
For over a decade, facilitating Research Use Only (RUO) CRISPR studies has resulted in an increasing number of CRISPR-based cell and gene therapies used to treat patients with debilitating diseases. In this webinar, learn more about the requirements for the development of CRISPR-based therapeutics and navigating the regulatory landscape.