Competitive Analysis of Guide RNA Formats

Contents
SNPs and the Need for Rapid, Amplification-Free Genetic Testing

More than 50% of disease-causing genetic mutations in humans are single nucleotide polymorphisms (SNPs). Tests for detecting single point mutations have been available for some time, but they require significant sample processing, infrastructure, expensive reagents, DNA amplification, and advanced optical equipment. This makes them costly and time-consuming, and impractical for mass-testing. The multiple bottlenecks throughout the process make early diagnosis challenging, and delays can lead to poorer patient outcomes.
Dr. Kiana Aran, CSO of Cardea Bio and Assistant Professor at KGI, aims to create novel solutions for unmet clinical needs, one of which is genetic testing. Dr. Aran’s innovative approach, the SNP-Chip, eliminates the need for excessive sample preparation and processing, amplification, technical staff, and expensive optics. The SNP-Chip is the first of its kind: an amplification-free, hand-held, point-of-testing device, capable of producing results from blood, saliva, or plant material with single-nucleotide specificity in under one hour. But how is the technology possible?
SNP-Chip: Combining Transistors, Graphene, and CRISPR Technology
Dr. Aran’s lab at KGI is well-known for its previous technological breakthrough, known as the CRISPR-Chip. First published in Nature Biomedical Engineering in 2019, CRISPR-Chip utilized catalytically deactivated Cas9 (dCas9) to detect target vs. non-target genes. Aran and her colleagues used the CRISPR-Chip to detect large insertions and deletions in genes - the commonly deleted exons in Duchenne Muscular Dystrophy patient samples. For a detailed explanation of how it works, you can read our article covering the original CRISPR-Chip technology.
Both SNP-Chip and CRISPR-Chip utilize three Nobel Prize-winning technological breakthroughs: transistors (Nobel Prize in Physics, 1956), graphene (Nobel Prize in Physics, 2010), and CRISPR-Cas9 (Nobel Prize in Chemistry, 2020). To most, it would seem extremely unlikely that transistors and CRISPR technologies would ever cross paths. To Dr. Aran, however, graphene provided the link that made it possible to apply transistors in the field of biology. Her chips utilizes next-generation graphene transistors, developed and mass-produced by Cardea Bio, which are highly sensitive and do not impact biological function. Biological interactions are rapid. When they dissipate through three-dimensional materials such as silicon, we can’t detect the entire signal because some of it gets lost in the material.
"Graphene is a single, thin sheet of carbon molecules, so the biological signals can be transferred much faster - we can see the signal almost in real-time. Graphene will open up a new era of applications for transistors, because now they can interact and communicate with biology.” "
- Dr. Aran explains why graphene is the key to using transistors for biological applications.
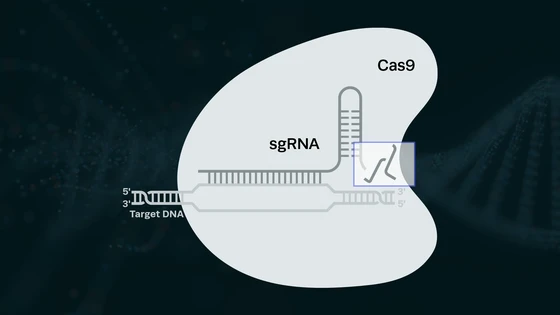
The SNP-Chip utilizes the power of CRISPR technology, not as genetic scissors, but as a search function. A chemical linker immobilizes the Cas enzyme on the graphene-coated surface of the transistor chip, where a Cas-gRNA complex is formed. Following calibration, unamplified DNA samples are incubated on the chip surface. The Cas nuclease can then search the DNA for the target specified by the gRNA - in the presence of a target-adjacent PAM, hybridization will occur, anchoring the target to the surface of the chip. DNA containing mismatches will not hybridize and will dissociate from the surface and be rinsed away before signal read-out.
The new paper explored the activity of three different Cas9 variants - dCas9, SpCas9, and MgaCas9 - to improve SNP discrimination. MgaCas9 is a recently described, highly sensitive Cas9 variant that uses a different PAM sequence to recognize potential targets, and it was this nuclease that allowed Aran’s team to achieve single-nucleotide specificity.
The group also examined different electrical measurements that could be taken from the chip, including current, capacitance, and surface potential, to determine the most sensitive parameters, as well as the effect of various buffers on signal output. The result is an elegant system capable of rapid, high-throughput detection of SNPs, all without the need for optics. You can hear Dr. Aran explain the significance of the SNP-Chip technology in her own words in this video.
Detecting Pathogenic SNPs in Sickle Cell Disease and Amyotrophic Lateral Sclerosis
To demonstrate the power of the SNP-Chip to detect SNPs, Dr. Aran and her team tested the technology on patients samples from two human disease models. Both sickle cell disease (SCD) and amyotrophic lateral sclerosis (ALS) are caused by SNPs in specific genes. In the case of SCD, this is the EV6 mutation in the HBB gene, and in ALS, it is the H44R mutation in the SOD1 gene. These diseases are currently key targets for CRISPR-based therapeutics, making them ideal models for testing the SNP-Chip.
Adapting the system to target different genetic diseases is achieved by simply designing new guide RNAs for each target. Like the original CRISPR-Chip paper, the SNP-Chip paper used Synthego-supplied synthetic guide RNAs to target the mutated regions in their genes of interest.
"We have had a great collaboration with Synthego since 2017, and we always use Synthego guide RNAs. In fact, a lot of our ideas for pipeline CRISPR products at Cardea Bio have come from our discussions with Synthego scientists."
- Dr. Kiana Aran, CSO of Cardea Bio and Assistant Professor at KGI
The SNP-Chip was able to detect the SNP markers for both SCD and ALS. More impressively, however, the MgaCas9 SNP-Chip was also able to discriminate between homozygous and heterozygous patient samples.
The Future of Genetic Testing with SNP-Chip

The power of SNP-Chip to detect pathogenic SNPs is certainly astounding, but the benefits don’t stop there. SNPs are also involved in aging, evolutionary processes, pharmacological interactions, and genotyping for agricultural use. Since the SNP-Chip can be easily reprogrammed to target different SNPs, it can be rapidly adapted for any of these applications. Upcoming iterations of the SNP-Chip are currently in development in order to increase the sensitivity of the chip while broadening its potential applications, such as detection of viral infections without amplification.
Dr. Aran says one of the key uses of the technology will be in CRISPR quality control. The technology cannot only detect but can also quantify the target DNA. This feature can be utilized to monitor and quantify the efficiency of CRISPR edits, such as those used as potential treatments for genetic diseases. The technology also offers the ability to monitor the extent of Cas interaction with guide RNAs, as well as the target DNA - information that can guide better CRISPR designs.
Looking further into the future, the team’s vision is to enable testing on a much larger scale. Graphene transistor technology is highly scalable, Dr. Aran explains, and the experiments in the SNP-Chip paper were performed using high-throughput systems developed at Cardea Bio. With the rapid pace of progress in the field of graphene transistors, she says it will eventually be possible to run thousands of tests on a single SNP-Chip device.
"When transistors were first invented in the 1950s, we had just a few transistors on a single chip and by the 1970s, we had 65,000 transistors on a single chip. In 10 years, we will have thousands of transistors on a single chip, and we can run thousands of experiments at once. "
- Dr. Kiana Aran, CSO of Cardea Bio and Assistant Professor at KGI