CRISPR-Cas9 gene editing has transformed cell and gene therapies, allowing scientists to treat the root cause of genetic disease by deleting and inserting genetic material. However, if you’re familiar with the CRISPR field, you’ve likely noticed a recent shift toward CRISPR methods that do not induce double-stranded breaks (DSBs) in DNA. These new technologies, such as base editing, prime editing, and epigenome editing, have been gaining popularity, and many are already in clinical use.
But why are these technologies necessary? What’s wrong with good old CRISPR-Cas9? And how can you edit DNA without cutting it, anyway?
Here, we will cover everything you need to know about new CRISPR technologies that modify DNA without DSBs. We’ll also discuss the need for safer therapies and the range of different derivative technologies that have been developed to address these issues.
The Need for Safer Gene Therapies
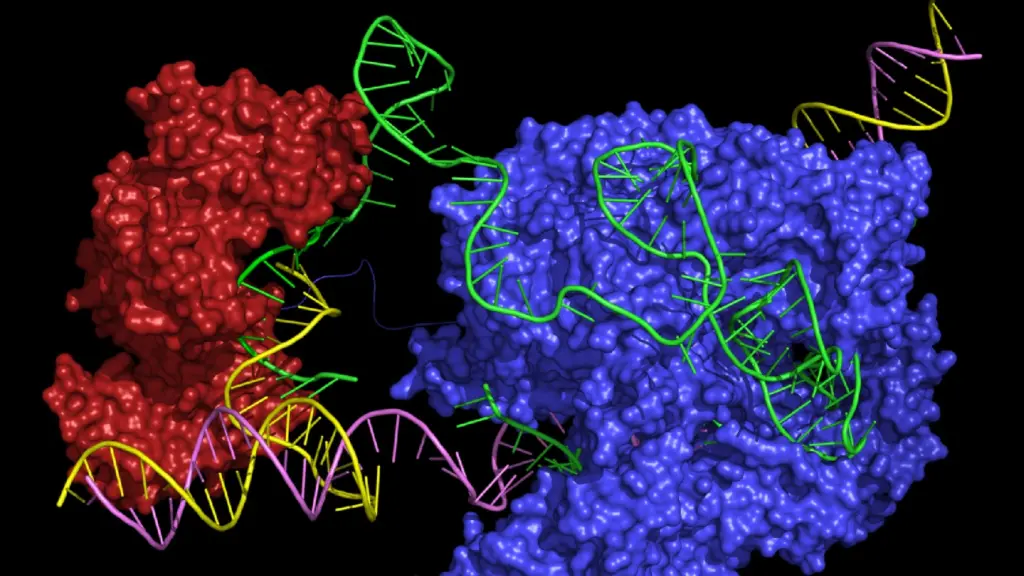
Compared to previous genome engineering technologies, the precision and efficiency of CRISPR-Cas9 edits make it the obvious choice for therapeutically altering the human genome. However, CRISPR-Cas9 is not a perfect system, especially since it is based on the creation of double-stranded breaks in the DNA double helix. The Cas9 nuclease acts as molecular scissors and cuts the DNA at the target site. The cell then repairs the break using either non-homologous end joining (NHEJ), which frequently causes indel mutations and can result in gene knockout, or homology-directed repair (HDR), which requires a DNA donor template and results in gene knock-in.
While it seems simple enough, the process of creating and repairing DSBs in DNA is fraught with risk. Any typical CRISPR editing study includes an analysis of off-target editing, yet the methods for detecting these depend on amplification and sequencing of the sites where this is likely to occur and therefore they may miss the bigger picture.
In recent years, there have been a plethora of studies dedicated solely to the exploration and mitigation of CRISPR-Cas9 safety issues. These include engineered Cas9 variants with higher editing fidelity, genetic profiling of entire cell populations following gene knockout, and detailed analysis of mismatch pairing, to name a few. However, there have also been many developments in CRISPR methods that do not induce DSBs. In the next few sections, we will dive into these newer methods.
Precision Editing
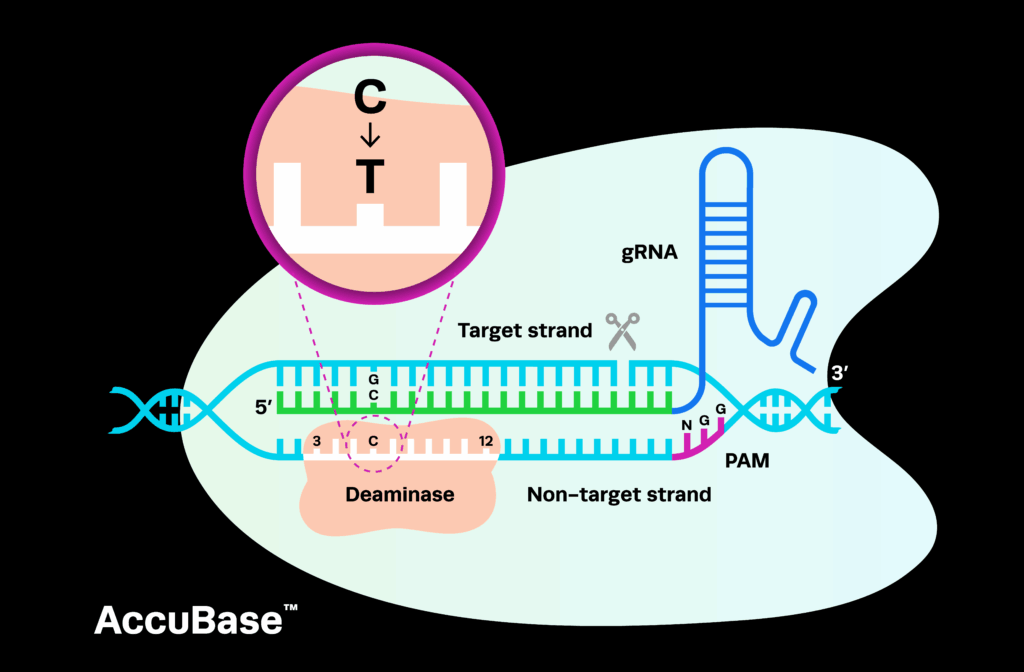
Base editing: nucleotide substitutions with bacterial deaminasesBase Editing is an advanced genome editing technique that precisely converts one base pair to another without introducing double-strand breaks in DNA. The process utilizes a catalytically inactive (dCas9) or nickase Cas protein fused to a DNA deaminase enzyme. These enzymes enable specific base conversions, such as C•G to T•A or A•T to G•C, via direct chemical modification of the DNA sequence. There are currently two types of base editing systems: cytidine deaminase base editors (CBEs) and adenine deaminase base editors (ABEs). Cytidine deaminases are naturally occurring enzymes isolated from bacteria that induce C to T substitutions in DNA. Adenine deaminases, however, do not occur naturally and were engineered from existing bacterial enzymes to induce A to G substitutions.
The majority of human genetic diseases are caused by single nucleotide polymorphisms, making base editors an ideal therapeutic option. Base editing is highly specific and minimizes undesired edits, off-target effects, or larger genomic rearrangements typically associated with double-strand breaks. However, challenges remain in the delivery of Base editing components to target cells, particularly in vivo, as well as in minimizing rare but potential off-target activity. Addressing these challenges is crucial for ensuring safe and effective use in therapeutic settings. Base Editing shows immense promise in treating genetic disorders caused by point mutations, including inherited blood disorders and metabolic diseases. It is being actively explored in preclinical studies and clinical trials, with the goal of translating its precision into impactful therapies for conditions that currently lack effective treatments.
For a deeper understanding of the mechanism, challenges, and its implications in cell and gene therapies, visit our Base Editing application page for full details.
Your base editing success starts with Synthego, where advanced engineering and precision converge in our Accubase™ Cytosine Base Editor (CBE). Offering high efficiency and exceptional fidelity, AccuBase is engineered to meet the demanding requirements of therapeutic development.
Base Editing is an advanced genome editing technique that precisely converts one base pair to another without introducing double-strand breaks in DNA. The process utilizes a catalytically inactive (dCas9) or nickase Cas protein fused to a DNA deaminase enzyme. These enzymes enable specific base conversions, such as C•G to T•A or A•T to G•C, via direct chemical modification of the DNA sequence. There are currently two types of base editing systems: cytidine deaminase base editors (CBEs) and adenine deaminase base editors (ABEs). Cytidine deaminases are naturally occurring enzymes isolated from bacteria that induce C to T substitutions in DNA. Adenine deaminases, however, do not occur naturally and were engineered from existing bacterial enzymes to induce A to G substitutions.
The majority of human genetic diseases are caused by single nucleotide polymorphisms, making base editors an ideal therapeutic option. Base editing is highly specific and minimizes undesired edits, off-target effects, or larger genomic rearrangements typically associated with double-strand breaks. However, challenges remain in the delivery of Base editing components to target cells, particularly in vivo, as well as in minimizing rare but potential off-target activity. Addressing these challenges is crucial for ensuring safe and effective use in therapeutic settings. Base Editing shows immense promise in treating genetic disorders caused by point mutations, including inherited blood disorders and metabolic diseases. It is being actively explored in preclinical studies and clinical trials, with the goal of translating its precision into impactful therapies for conditions that currently lack effective treatments.
For a deeper understanding of the mechanism, challenges, and its implications in cell and gene therapies, visit our Base Editing application page for full details.
Your base editing success starts with Synthego, where advanced engineering and precision converge in our Accubase™ Cytosine Base Editor (CBE). Offering high efficiency and exceptional fidelity, AccuBase is engineered to meet the demanding requirements of therapeutic development.
Accubase™ Cytosine Base Editor
Available in Research-grade and GMP, RNP AccuBase base editor delivers high efficiency and exceptional fidelity, ensuring precise single base modifications with minimal off-target activity. Designed to meet stringent therapeutic development standards, AccuBase is a safe and reliable solution for advancing clinical and commercial applications.
Prime and twin prime editing: insertions and deletions
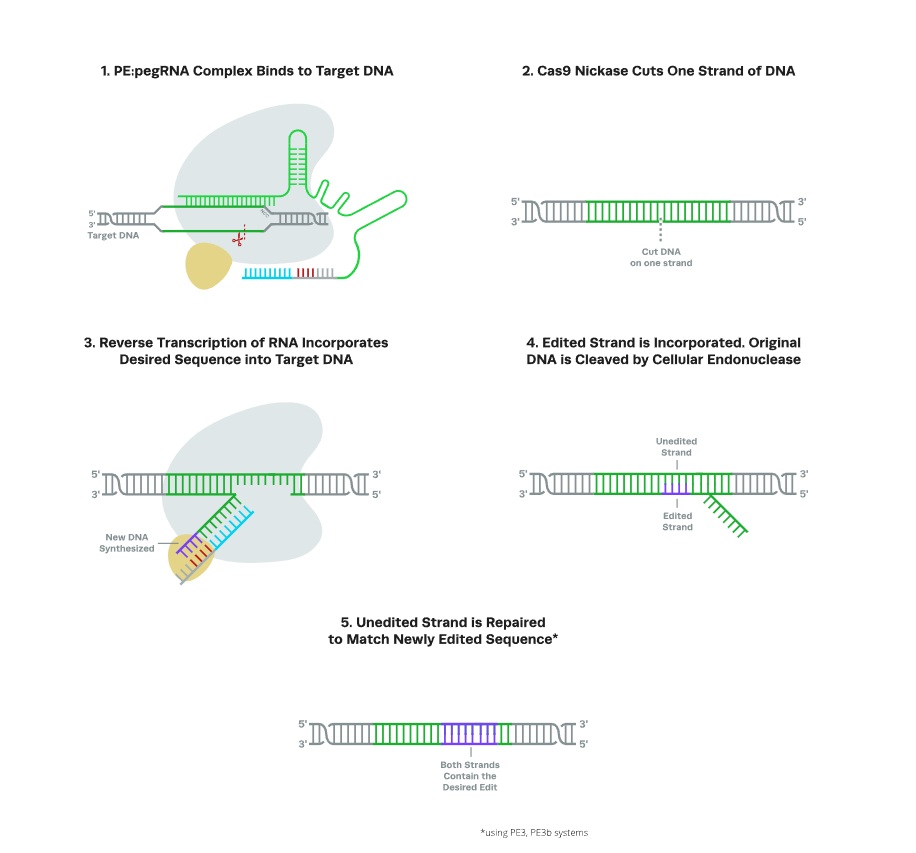
Prime editing systems allow for both substitution mutations and the insertion and deletion of small fragments of DNA without DSBs. Prime editors (PEs) use an engineered Cas9 nickase (nCas9), which cuts only one strand of DNA, fused to reverse-transcriptase (RT), a viral enzyme that allows for reverse transcription of a DNA template, effectively writing a new section of DNA into the genome. Unlike CRISPR-Cas9, which uses an sgRNA to direct editing and a DNA donor template for HDR, PE systems employ a single construct known as a prime editing guide RNA (pegRNA).
The engineered pegRNA construct contains both the primer binding site (PBS) to direct editing and a DNA sequence that contains the edit; the Cas9 nickase binds at the PBS and cuts one strand of DNA, and the RT uses the other part of the pegRNA sequence as a template for reverse transcription, adding the desired nucleotides to the nicked strand. The original DNA sequence is then removed by endonucleases, and an additional guide RNA directs nCas9 to nick the opposing (unedited) strand, causing the cell to repair the DNA using the edited strand as a template.
The original PE system allowed for insertions of up to 50 base pairs and deletions of up to 80 base pairs. A newer iteration, twin-prime editing (twinPE), uses two pegRNAs to simultaneously target opposing strands of DNA, allowing for the insertion and deletion of much larger sequences. PE systems show promise in the treatment of many genetic disorders, including heart disease, sickle cell disease, prion diseases, diabetes type II, and Alzheimer’s, while twinPE can potentially be used to treat diseases caused by multiple pathogenic mutations in large genes.
Dive deeper into the mechanisms, challenges, and clinical uses of prime editing on our Prime Editing application page.
Prime Editing Enables Higher Fidelity Gene Editing in Mice Than Traditional CRISPR Methods

Controlling Gene Expression with Engineered Cas Nucleases
The first CRISPR derivatives used to make genetic modifications without inducing DSBs were the CRISPRactivation (CRISPRa) and CRISPRinhibition (CRISPRi) systems. These technologies use a catalytically dead Cas9 (dCas9), which retains its search function, but has been inactivated so that it can no longer cleave DNA.
CRISPRi originally worked via directing dCas9 to the promoter of a target gene, where it will bind and prevent transcription of the gene, leading to gene silencing. This activity was later enhanced by fusing dCas9 with other effectors that help to repress transcription. In contrast, CRISPRa uses dCas9 fused to transcriptional activators, directing them to gene promoters or enhancers to increase transcription.
However, CRISPRi and CRISPRa can only regulate the expression of genes that are already present in the genome and are not capable of correcting disease-causing mutations. Using CRISPR to actually modify the underlying genetic sequence without creating DSBs was more challenging, and required the use of other types of proteins from bacteria and viruses to work.
CRISPR, But Different: Next-Generation Gene Therapy Methods
There now exist a variety of CRISPR derivatives that can be used to alter DNA, RNA, and the epigenome for therapeutic purposes without inducing DSBs. In this section, we’ll explore how these technologies work and their potential applications in the treatment of disease.
RNA editing: using new Cas nucleases to alter the transcriptomeOne of the interesting discoveries when scientists started exploring other bacterial CRISPR systems was new Cas nucleases that could target RNA instead of DNA. The ability to edit RNA offers an interesting alternative to traditional CRISPR because RNA is transiently expressed; any changes to RNA inside a cell are not permanent or heritable. There are many RNA-mediated diseases in humans caused by defects in the splicing, editing, modification, or translation of RNA transcripts which can potentially be treated using RNA editing. Viruses with RNA genomes, including SARS-CoV-2, can also be targeted using RNA editing.
The first RNA-targeting Cas nucleases to be described were the Cas13 family, which includes many subtypes. However, Cas13 cannot be used for therapeutic editing because of its problematic ‘collateral activity’; once it recognizes and cuts its target transcript, it tends to destroy all other cellular transcripts, causing cell death. Cas13 is now primarily used for diagnostic purposes, including for the rapid detection of the SARS-CoV-2 virus and other RNA pathogens.
The more recent discovery of Cas7-11 has led to renewed interest in RNA editing for therapeutic purposes. Cas7-11 displays highly specific RNA editing with no collateral activity. Importantly, it is also more efficient than other RNA knockdown methods, such as short hairpin RNA (shRNA), and with less impact on cell viability. This new nuclease provides an avenue for the treatment of RNA-mediated diseases for which there are limited treatment options, including Huntington’s disease. It can also potentially be used for the therapeutic removal of RNA viruses from human cells.
One of the interesting discoveries when scientists started exploring other bacterial CRISPR systems was new Cas nucleases that could target RNA instead of DNA. The ability to edit RNA offers an interesting alternative to traditional CRISPR because RNA is transiently expressed; any changes to RNA inside a cell are not permanent or heritable. There are many RNA-mediated diseases in humans caused by defects in the splicing, editing, modification, or translation of RNA transcripts which can potentially be treated using RNA editing. Viruses with RNA genomes, including SARS-CoV-2, can also be targeted using RNA editing.
The first RNA-targeting Cas nucleases to be described were the Cas13 family, which includes many subtypes. However, Cas13 cannot be used for therapeutic editing because of its problematic ‘collateral activity’; once it recognizes and cuts its target transcript, it tends to destroy all other cellular transcripts, causing cell death. Cas13 is now primarily used for diagnostic purposes, including for the rapid detection of the SARS-CoV-2 virus and other RNA pathogens.
The more recent discovery of Cas7-11 has led to renewed interest in RNA editing for therapeutic purposes. Cas7-11 displays highly specific RNA editing with no collateral activity. Importantly, it is also more efficient than other RNA knockdown methods, such as short hairpin RNA (shRNA), and with less impact on cell viability. This new nuclease provides an avenue for the treatment of RNA-mediated diseases for which there are limited treatment options, including Huntington’s disease. It can also potentially be used for the therapeutic removal of RNA viruses from human cells.
PASTE editing: inserting large DNA segmentsProgrammable Addition via Site-specific Targeting Elements (PASTE) is one of the latest additions to the CRISPR toolbox, harnessing the power of prime editors and building on them. PASTE uses bacteriophage serine integrase proteins to insert up to 36 kb of DNA into the genome. Cleverly, the PASTE system employs prime editors to integrate DNA sequences known as integrase landing sites (AttB), which the integrase protein attachment site (AttP) will locate and bind to for editing. Using PEs to integrate the AttB sites, PASTE can potentially be used for targeted large-scale gene knock-in anywhere in the human genome.
The PASTE method could have enormous therapeutic value in the treatment of genetic diseases caused by many pathogenic mutations in large genes. For example, X-linked agammaglobulinemia (XLA) is caused by a variety of mutations in the Bruton's tyrosine kinase (BTK) gene, and researchers have struggled to develop a CRISPR-Cas9 gene therapy for this disease based on the size of the required DNA donor template for repair. PASTE may offer an alternative to correct the mutated gene with relative ease, without inducing potentially harmful DSBs.
Programmable Addition via Site-specific Targeting Elements (PASTE) is one of the latest additions to the CRISPR toolbox, harnessing the power of prime editors and building on them. PASTE uses bacteriophage serine integrase proteins to insert up to 36 kb of DNA into the genome. Cleverly, the PASTE system employs prime editors to integrate DNA sequences known as integrase landing sites (AttB), which the integrase protein attachment site (AttP) will locate and bind to for editing. Using PEs to integrate the AttB sites, PASTE can potentially be used for targeted large-scale gene knock-in anywhere in the human genome.
The PASTE method could have enormous therapeutic value in the treatment of genetic diseases caused by many pathogenic mutations in large genes. For example, X-linked agammaglobulinemia (XLA) is caused by a variety of mutations in the Bruton's tyrosine kinase (BTK) gene, and researchers have struggled to develop a CRISPR-Cas9 gene therapy for this disease based on the size of the required DNA donor template for repair. PASTE may offer an alternative to correct the mutated gene with relative ease, without inducing potentially harmful DSBs.
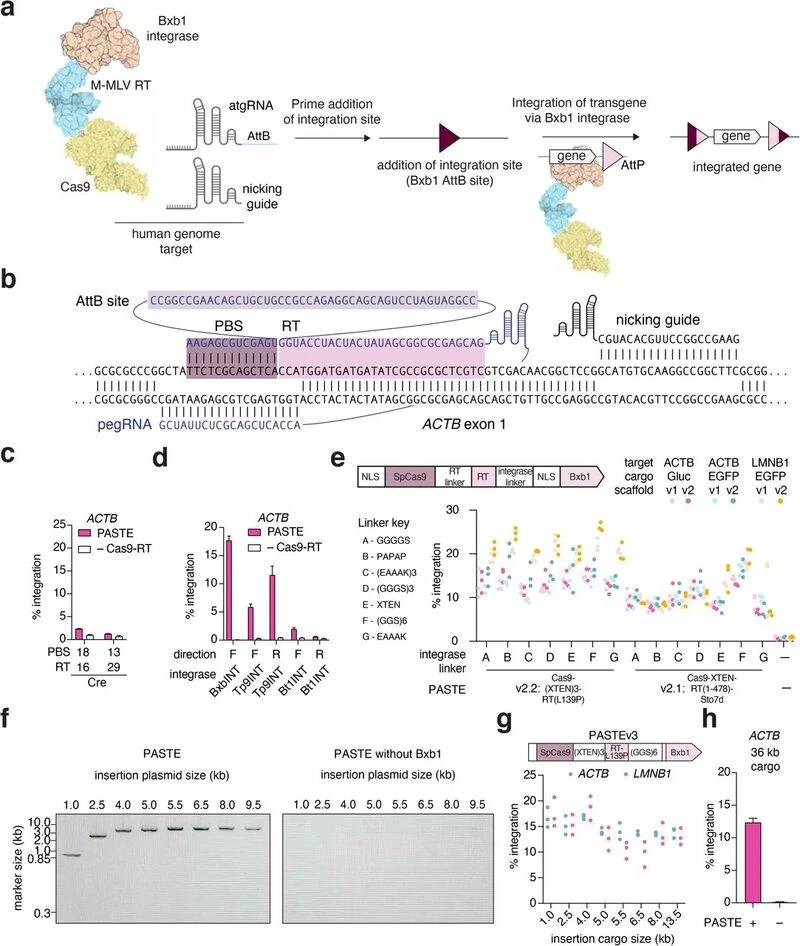
PASTE editing allows for programmable gene insertion without double-stranded breaks, independent of DNA repair pathways. Image from paper.
Epigenome editing: switching genes on and offCRISPR-based epigenetic editing is another nascent method for therapeutics without DSBs. The epigenome controls how our genes are expressed through DNA methylation (5mC), histone modifications, and non-coding RNAs. Because 5mC and histone modifications are regulated by enzymes, such as DNA methyltransferases and histone acetyltransferases, dCas9 can be fused to epigenetic enzymes or their catalytic domains in order to alter the epigenome at a target site, switching gene expression on or off.
For example, if a gene is transcriptionally repressed by 5mC in the CpG islands of the gene promoter, this epigenetic mark can be oxidized by fusing dCas9 to the catalytic domain of a ten-eleven translocation dioxygenase, such as TET1CD, restoring expression. In contrast, if a gene is upregulated due to the addition of histone 3 lysine 27 acetylation (H3K27ac), dCas9 fused to histone deacetylase 3 (HDAC3) can be used for targeted deacetylation, reducing the expression of the gene.
This type of CRISPR-based epigenetic reprogramming offers the ability to switch genes on and off, with a range of potential therapeutic applications. Although there are no current trials, there have been many proof-of-concept and pre-clinical studies demonstrating the power of these methods. For example, epigenetic editing can upregulate fetal hemoglobin production in adults who suffer from sickle cell disease. Similarly, it can restore the expression of genes repressed by hypermethylation, as seen in the FMR1 gene which causes Fragile X syndrome.
Repressive epigenetic marks can also be removed in order to alleviate neuropsychiatric disorders, such as anxiety and alcohol use disorder, caused by excessive alcohol exposure during adolescence. Additionally, epigenetic editing has enormous potential in cancer therapeutics by correcting forms of epigenetic dysregulation that contribute to tumor growth.
CRISPR-based epigenetic editing is another nascent method for therapeutics without DSBs. The epigenome controls how our genes are expressed through DNA methylation (5mC), histone modifications, and non-coding RNAs. Because 5mC and histone modifications are regulated by enzymes, such as DNA methyltransferases and histone acetyltransferases, dCas9 can be fused to epigenetic enzymes or their catalytic domains in order to alter the epigenome at a target site, switching gene expression on or off.
For example, if a gene is transcriptionally repressed by 5mC in the CpG islands of the gene promoter, this epigenetic mark can be oxidized by fusing dCas9 to the catalytic domain of a ten-eleven translocation dioxygenase, such as TET1CD, restoring expression. In contrast, if a gene is upregulated due to the addition of histone 3 lysine 27 acetylation (H3K27ac), dCas9 fused to histone deacetylase 3 (HDAC3) can be used for targeted deacetylation, reducing the expression of the gene.
This type of CRISPR-based epigenetic reprogramming offers the ability to switch genes on and off, with a range of potential therapeutic applications. Although there are no current trials, there have been many proof-of-concept and pre-clinical studies demonstrating the power of these methods. For example, epigenetic editing can upregulate fetal hemoglobin production in adults who suffer from sickle cell disease. Similarly, it can restore the expression of genes repressed by hypermethylation, as seen in the FMR1 gene which causes Fragile X syndrome.
Repressive epigenetic marks can also be removed in order to alleviate neuropsychiatric disorders, such as anxiety and alcohol use disorder, caused by excessive alcohol exposure during adolescence. Additionally, epigenetic editing has enormous potential in cancer therapeutics by correcting forms of epigenetic dysregulation that contribute to tumor growth.
The Future of CRISPR Without Cutting DNA

In the decade since CRISPR was established as a tool for gene editing, the field has made astounding progress, particularly in terms of therapeutic application. Yet the public perception of gene editing in humans is still dubious at best, partly due to a lack of education and awareness on this issue. Moreover, there remain several key safety issues associated with any use of traditional CRISPR in human patients.
While traditional CRISPR-Cas9 will always be a mainstay in the field of gene editing, the toolbox continues to expand and improve for therapeutic use. CRISPR derivatives that are able to control the expression of genes or modify the genetic code without inducing DSBs are not only a promising alternative for therapy because of their increased safety profiles, but are also likely to increase positive public perceptions of these therapies.
Many of these technologies are currently approaching clinical translation, and base editors are already being tested in clinical trials; as evidence of their potential mounts, we’re likely to see a more marked shift towards these DSB-free technologies for gene therapy in the coming years. Current regulatory trends and FDA initiatives in support of gene therapy development have also contributed to a more hopeful atmosphere in the CRISPR therapy community. With continued progress, these positive changes will hopefully lead to an increase in the successful clinical translation of CRISPR therapies.
We hope this blog helped to familiarize you with all the CRISPR derivatives for DSB-free genetic modification and why they’re changing the landscape of gene therapy. At Synthego, we not only provide large-scale GMP-grade reagents for safer and more efficient CRISPR gene therapies, but we’re also committed to CRISPR education and outreach. For more of the latest CRISPR news and breakthroughs, subscribe to The Bench blog!